Current Concepts in Imaging Asymptomatic Hematuria
Main Article Content
Abstract
Hematuria is a very common clinical condition that most clinicians will encounter. Hematuria is divided into gross and microscopic hematuria with a wide variety of etiologies. The imaging evaluation of asymptomatic hematuria is generally tailored to identifying a common cause (urinary tract stones) and the most worrisome cause (carcinoma). The workup for asymptomatic gross hematuria is generally agreed upon and includes both CT and cystoscopy. However, the imaging evaluation of asymptomatic microscopic hematuria is controversial due to the much lower risk of urinary tract malignancies. The purpose of this article is to review the imaging of the upper urinary tract focusing on CT, MRI, and ultrasound. Current European and North American society guidelines, imaging techniques, typical imaging findings, diagnostic accuracy, and risks will all be addressed highlighting the current knowledge gaps, particularly between CT and ultrasound.

1. Background
Hematuria is a common indication for urological evaluation, resulting in up to 2 million urology referrals in the United States each year.1 The prevalence of hematuria ranges from 0.19 to 31% in the literature, depending on the definition of hematuria, population demographics, and diagnostic testing approaches.2,3 Hematuria can be classified as gross hematuria (also referred to as macrohematuria) or microhematuria. Gross hematuria is defined as red blood cells resulting in readily visible red or brown coloration of the urine. The American Urological Association (AUA) defines microhematuria as ≥3 red blood cells per high-power field.2 For this review, we will examine the clinical evaluation and diagnostic imaging pertaining to asymptomatic hematuria focusing closely on the controversies in microscopic hematuria.
2. Etiologies
Hematuria may originate from the renal parenchyma or anywhere along the urinary tract, which can be subdivided between glomerular or nonglomerular pathologies. Potential etiologies include malignancy, glomerulonephropathy, urinary tract infection, inflammation, medication effect, benign prostatic hyperplasia, urinary calculus, or anatomic anomalies.2 Gynecologic sources of bleeding may also be mistakenly attributed to hematuria. Microhematuria is also frequently discovered in young adults, and the association with vigorous exercise is well documented.4
As opposed to gross hematuria, microhematuria is often incidentally discovered and associated with lower rates of urinary tract malignancy and diagnostic yield.5-9 One prospective study of 4020 patients with hematuria (with urinary tract infection excluded) demonstrated a prevalence of urinary tract malignancy of 18.9% for gross hematuria while only 4.8% for microscopic hematuria.6 Meanwhile, the prevalence of urinary stones was roughly equal in the two hematuria groups with 7.8% in microscopic and 8.8% in macroscopic hematuria.6 No identifiable cause was found in over 75% of patients. In a contemporary retrospective study, the incidence of bladder cancer was 11% and renal cancer 1.4% in 2311 patients with gross hematuria, compared to 2.7% and 0.4% respectively in 1245 patients with microscopic hematuria.10 Additionally, the incidence of upper tract urothelial cancer was also higher in patients with gross hematuria at 0.8%, while no cases were observed in patients with microhematuria.10 The most recent AUA guidelines cited the aggregate rate (taken from multiple studies spanning 2010 to 2019) for urinary tract malignancy in the setting of microhematuria is 1%.2
3. Diagnostic Evaluation
3.1 Society guidelines
There is a consensus that the diagnostic work-up of gross hematuria should consist of urinary tract imaging (usually CT) and cystoscopy, however, the evaluation of microhematuria remains controversial given the paucity of high-quality evidence and heterogeneity of recommendations reflected in the literature and societal guidelines.11 According to AUA guidelines, the presence of microhematuria can be identified on formal urinalysis with ≥3 red blood cells per high-power field on microscopy from a single urine sample.2 Although the potential etiologies for microhematuria often consist of clinically insignificant or non-malignant entities including medical renal disease, gynecologic causes, kidney stones, urinary tract infections, strictures, and benign prostatic hyperplasia, much of the diagnostic testing aims to detect urinary tract malignancy. The AUA definition of microhematuria was found to have 50% sensitivity, 84% specificity, and 1.3% positive predictive value for urologic cancer.12 Multiple studies questioned the diagnostic utility in evaluating for urinary tract cancer in patients under 40 years of age with asymptomatic microhematuria, citing exceedingly low rates of cancer detection.5,12-16
Women carry a lower risk for urologic malignancies compared to men and exhibit female-specific conditions such as menstruation, pelvic organ prolapse, and urogenital atrophy that may confound hematuria detected on urine specimen.17 One large retrospective population-based study of 156,691 patients with hematuria showed an incidence of urologic cancer for patients less than 40 years old to be 0.07% in women compared to 0.43% in men.12 These findings were reproduced in another retrospective study of 3,573 women, where individuals under the age of 60 who did not have a history of smoking or present with gross hematuria did not exceed urologic cancer rates of 0.6%.18 A committee formed by the American College of Obstetricians and Gynecology and the American Urogynecologic Society recommended that low-risk, never-smoking women with asymptomatic hematuria should only undergo clinical evaluation if there are greater than 25 red blood cells per high power field.17 Furthermore, the most recent AUA guidelines released in 2020 include sex-specific guidelines in an attempt to incorporate this known decrease in risk for urinary tract cancer in women.2
According to the AUA 2020 guidelines, when evaluating a patient with confirmed microscopic hematuria on urinalysis, a complete history, and physical exam should be obtained with particular attention to risk factors for urologic malignancy, history of anticoagulation, or medical renal disease. Patients with findings consistent with a medical renal disease such as renal insufficiency, proteinuria, or cellular casts on urine microscopy should be referred to a nephrologist for further evaluation. Significant risk factors for urothelial malignancy include smoking history, male sex, history of gross hematuria, occupational exposures (benzene chemicals or aromatic amines), prior pelvic radiation, and positive family history of urothelial cancer or Lynch Syndrome.2 Similar guidelines for history-taking practices are reflected in other guidelines such as the Canadian Urological Association and the Dutch Association of Urology. The AUA has proposed a risk stratification system that groups patients into low-, intermediate-, and high-risk groups that incorporate patient gender, age, smoking history, degree of hematuria, and risk factors for urothelial cancer (Table 1).
Table 1: AUA Microhematuria Risk Stratification
Although limited evidence currently exists in the evaluation of patients in the low-risk category, rates of urologic malignancy in patients without risk factors is very low especially in light of low overall cancer rates in the microhematuria population as a whole. The benefits of identifying the cause of microhematuria have to be weighed with the risks of undergoing diagnostic testing with imaging and/or cystoscopy. Under the most recent AUA guidelines, patients who identify as low risk are encouraged to engage in shared decision-making with clinicians and determine whether to have a repeat urinalysis in 6 months or proceed directly to cystoscopy and renal ultrasound. Renal ultrasound and cystoscopy are also recommended for patients in the intermediate-risk category. Lastly, patients who meet the criteria for high risk such as age ≥ 60 years or with >30 pack-year smoking history should undergo cystoscopy and upper urinary tract imaging with multiphasic CT urography (CTU). Patients who have contraindications to CTU can alternatively be evaluated with MR urography (MRU) versus retrograde pyelography along with renal ultrasound or unenhanced axial imaging. On the other hand, the Canadian Urological Association and the Dutch Association of Urology guidelines recommend ultrasonography as the first-line imaging test of the upper urinary tract, while CTU may be reserved for cases with abnormal or inconclusive findings.11,19 The European Urological Association (EUA) does not have explicit guidelines for the management of hematuria, however, recommendations are made for specific malignancies where hematuria may be a presenting symptom.20 For example, the EUA recommends CTU as the initial diagnostic imaging study for upper urinary tract urothelial carcinoma, provided the patient meets the associated risk factors.20
3.2 Role of Imaging
3.2.1 Background
We will discuss the current repertoire of imaging modalities utilized in the investigation of hematuria including ultrasound, computed tomography, magnetic resonance imaging, and retrograde pyelography. Intravenous urography (a.k.a. intravenous pyelography) was once considered the gold standard of urinary tract imaging, which consisted of acquiring radiographs at timed intervals with varying patient positions following the administration of iodinated intravenous contrast to interrogate the upper urinary tracts and bladder. This technique is now largely replaced by CTU and no longer recommended by the American College of Radiology (ACR) for first-line evaluation of hematuria.21 The ACR also does not recommend conventional abdominal radiographs (KUB) nor conventional CT abdomen and pelvis with IV contrast; therefore, we will not explore these imaging modalities in detail. The primary goal of imaging in the evaluation of microscopic hematuria is to rule out urologic malignancy, however, a range of benign etiologies especially urinary stones may also be detected with imaging techniques.
3.2.2 CT Urography
CT urography (CTU) takes advantage of cross-sectional imaging and contrast enhancement of the urinary collecting system to fully assess the kidneys, ureter, and bladder in a single examination. The advent of multidetector computed tomography (MDCT) has afforded rapid imaging acquisition of the entire urinary tract in a single breath-hold. Thin-section (≤ 3mm) images of the abdomen and pelvis can be obtained in the axial plane and reformatted for sagittal and coronal reformatted images. This multi-planar assessment of the urinary tracts can precisely delineate anatomic relationships which were previously unachievable with traditional intravenous urography. Therefore, intravenous urography has largely been supplanted by CTU for the evaluation of hematuria by institutions with access to MDCT with relatively similar or even reduced radiation dose.
CTU is a multi-phase exam of the abdomen and pelvis that typically consists of unenhanced, nephrographic, and excretory phases, although no standardized universal protocol currently exists.22-23 An unenhanced phase is optimized for the evaluation of urinary tract calculi and to provide a baseline unenhanced appearance of urologic masses that can be further characterized on subsequent contrast-enhanced series. The nephrographic phase is obtained approximately 100 seconds after the administration of intravenous iodinated contrast to best identify renal masses that would otherwise be less conspicuous on an unenhanced exam. Finally, an additional excretory phase acquisition is performed 5-15 minutes after administration of contrast to opacify the urinary collecting system to evaluate the urothelium. Some institutions may include an additional corticomedullary phase which is obtained 20-30 seconds after IV contrast injection, however, local practice patterns may vary.19,22 Various adjunctive techniques may also be implemented to augment distension and opacification of the urinary tract such as oral or intravenous hydration or the administration of intravenous furosemide.23
Several techniques have been implemented to reduce radiation in CTU exams. A split-bolus technique is frequently used which obtains both the nephrographic and excretory phases in a single phase, thus reducing the number of total acquisitions and radiation exposure. In addition to reducing the number of phases in CTU, iterative reconstruction techniques of each scan phase also significantly decrease radiation exposure while maintaining image quality.22-24 Finally, dual-energy CT is an emerging technique that can create virtual unenhanced imaging from a single contrast-enhanced acquisition.25
Unenhanced CT is important in the evaluation of hematuria as it is the optimal imaging modality for the evaluation of urinary calculi, with sensitivity estimated at >95%.21 Unenhanced CT is exquisitely sensitive for small stones, as well as providing accurate information on stone morphology and location throughout the urinary tract. The main disadvantage compared to alternative techniques such as renal ultrasound and abdominal radiography is the higher radiation dose. Radiation dosing for conventional unenhanced CT examinations of the abdomen pelvis is estimated at 9.9 mGy, which can be reduced substantially (1.9 mGy) with low-dose techniques without sacrificing sensitivity and specificity for urinary calculi.21 Low-dose techniques can also be adopted for the initial unenhanced phase for a CT urography exam to further minimize radiation exposure.19
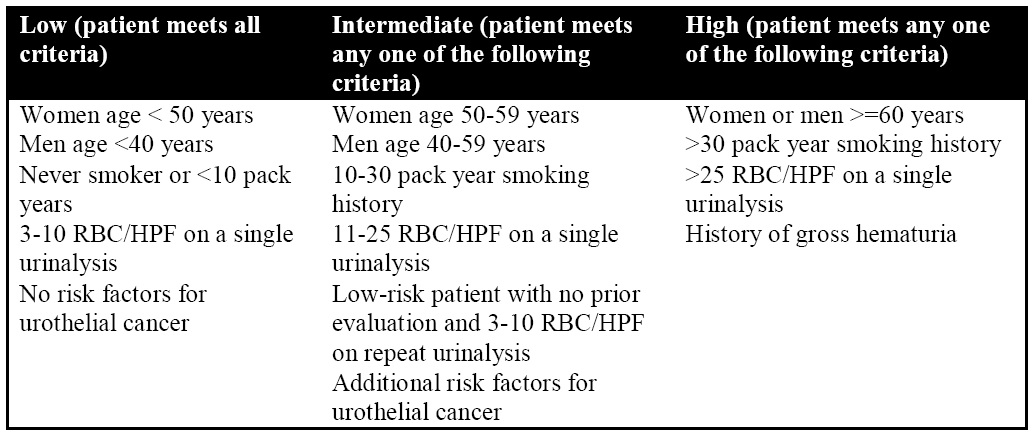
CT is often the imaging modality of choice for the evaluation of renal masses with comprehensive lesion characterization with unenhanced and contrast-enhanced acquisitions. Renal cell carcinoma, particularly the clear cell subtype, typically presents as a soft tissue mass originating from the renal cortex that enhances, defined as a change of 20 Hounsfield Units or more between unenhanced and nephrographic phases (Figure 1).26 There may be associated calcifications and internal heterogeneity that are compatible with necrosis. CT is also invaluable in determining the extent of locoregional disease spread such as renal vein invasion, lymphadenopathy, or regional metastases (Figure 2).
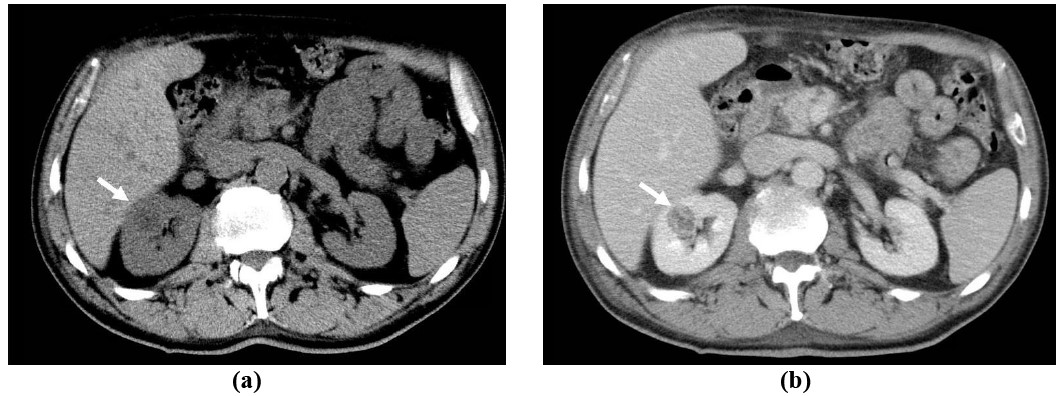
Figure 1: 70-year-old male with incidentally discovered right renal mass: Axial unenhanced (a) and nephrographic phase (b) CT images of the abdomen demonstrates an enhancing mass (> 20 Hounsfield Unit increase) in the right kidney, consistent with renal cell carcinoma (white arrow).
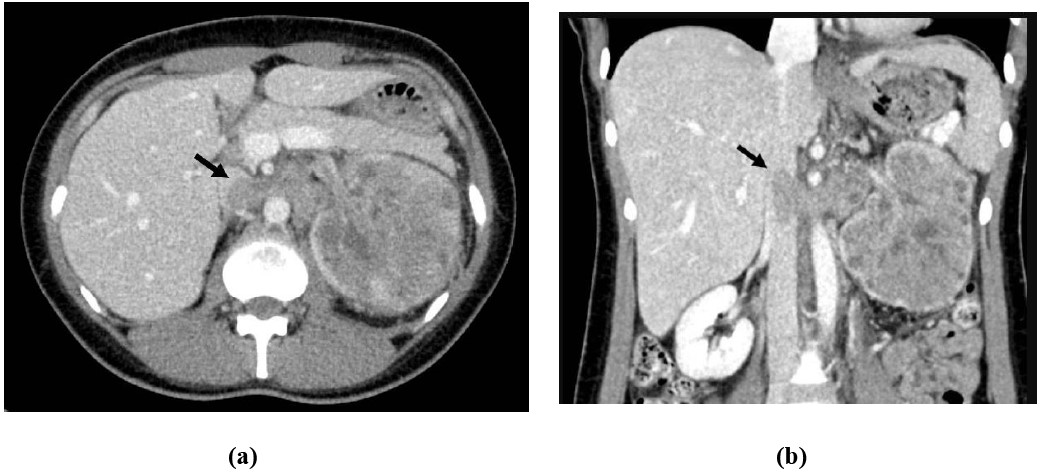
Figure 2: 33-year-old female presents with abdominal pain and gross hematuria: Axial (a) and coronal (b) contrast-enhanced CT images of the abdomen demonstrates a large left renal mass with left renal venous invasion extending into the inferior vena cava, consistent with renal cell carcinoma (black arrow).
CTU is more sensitive and specific compared to intravenous urography with a pooled sensitivity of 96% and a pooled specificity of 99% for detecting upper urinary tract urothelial malignancies.27 Urothelial cell carcinoma (UCC; previously known as transitional cell carcinoma) is the most common primary renal malignancy after renal cell carcinoma.28 UCC of the upper urinary tract presents as a focal intraluminal mass, sometimes with mucosal extension resulting in mural thickening (Figure 3). Infiltrative UCC may demonstrate centrifugal extension from its urothelial source with obscuration of the renal sinus fat and invasion of the renal parenchyma (Figure 4).29 Ureteral filling defects on CTU that exhibit soft-tissue attenuation should raise the suspicion for malignancy such as UCC and prompt direct ureteroscopic visualization (Figure 5).
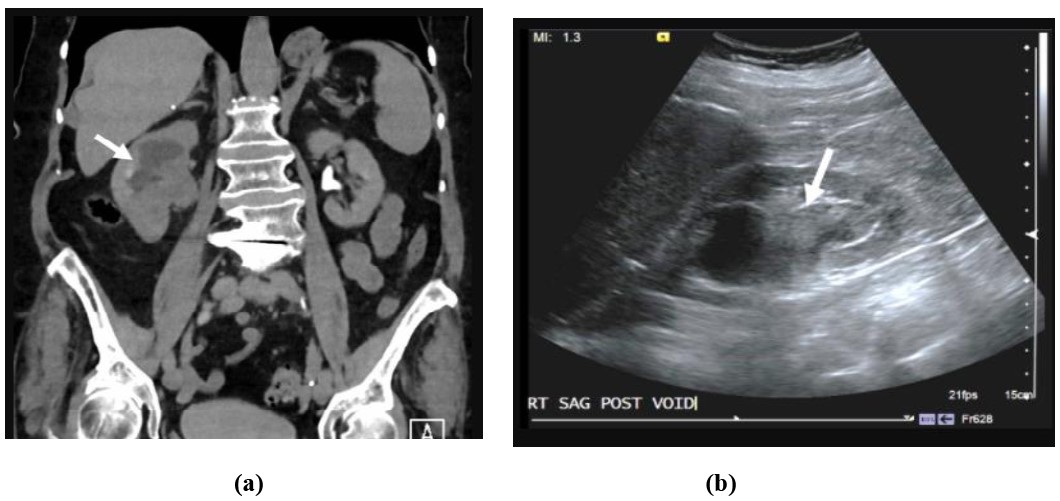
Figure 3: 79-year-old female with gross hematuria: Coronal CT urogram (a) and sagittal ultrasound of the right kidney (b) shows polypoid intraluminal mass (white arrow) centered in the renal pelvis resulting in hydronephrosis. Pathology was consistent with urothelial cell carcinoma.
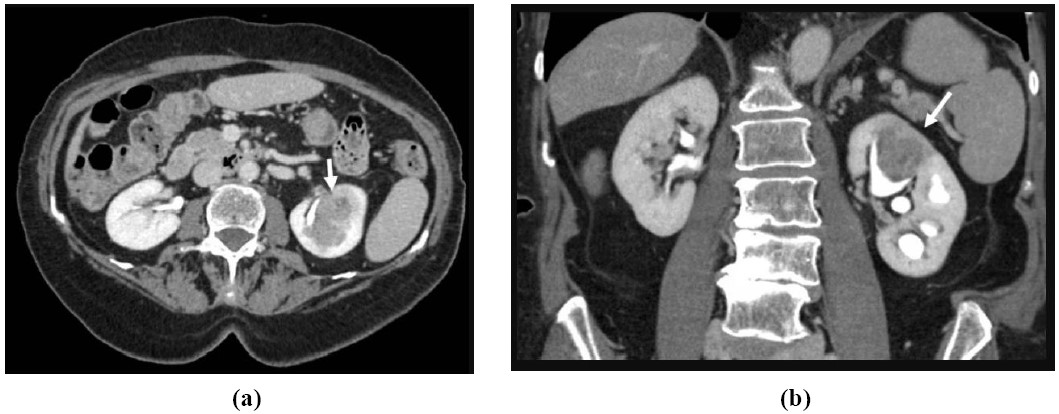
Figure 4: 81-year-old female with gross hematuria and left flank pain: Axial (a) and coronal (b) CTU images of the kidneys shows an infiltrative mass (white arrow) centered within an upper pole calyx in the left kidney with extension into the renal cortex. Pathology was consistent with urothelial cell carcinoma.
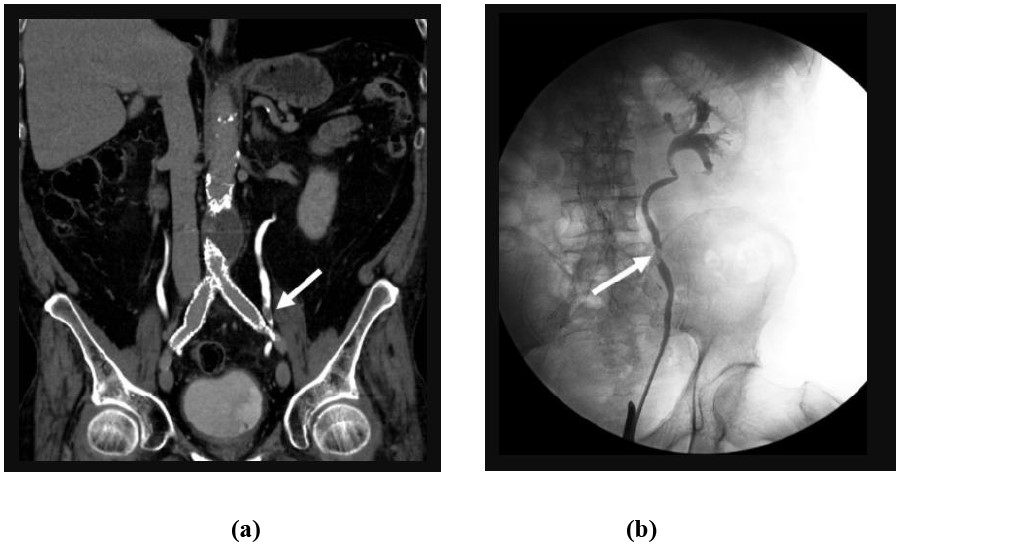
Figure 5: 79-year-old male with gross hematuria: Coronal CTU (a) and retrograde pyelogram (b) images demonstrates a focal filling defect (white arrow) within the left mid-ureter consistent with urothelial cell carcinoma.
UCC occurs far more frequently in the urinary bladder compared to the renal pelvis and ureter, and it also accounts for the majority of urologic malignancy detected in patients with microhematuria. Although CTU has shown to be up to 86.3% sensitive and 92.4% specific for the detection of bladder cancer, cystoscopy remains the diagnostic gold standard and a key component of microhematuria evaluation for intermediate- and high-risk groups.2,30 Bladder UCC can be seen on CTU as a focal intraluminal filling defect or mass (Figure 6), however inadequate bladder distension or opacification may limit assessment.
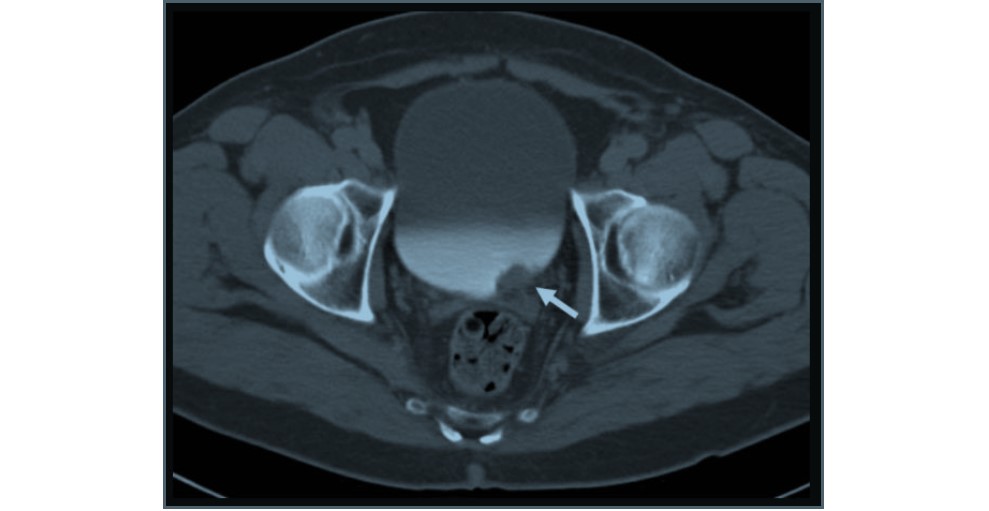
Figure 6: 71-year-old male with gross hematuria: Axial CTU of the bladder shows an intraluminal mass (white arrow) in the urinary bladder that overlies the left ureteral orifice consistent with urothelial cell carcinoma.
Given the widespread adoption of CTU in lieu of intravenous urography, the AUA previously released guidelines in 2012 recommended that all patients over the age of 35 with asymptomatic microhematuria undergo cystoscopy and radiologic evaluation with CTU or MRU to rule out urologic malignancy.31 Studies have since demonstrated that while CTU is the most comprehensive study to evaluate the urothelium, the probability of urothelial malignancy in microhematuria is very low especially in patients younger than 50 years of age with one study finding no malignancies in 442 patients with at most a theoretical 1% risk.32 A simulation model analyzing the costs of hematuria workup for patients aged 35 years or older showed uniform CT imaging was associated with higher health care costs, false positives, and radiation exposure with only marginally increased cancer detection.1 One caveat of the aforementioned analysis is that the estimate of 91% ultrasound sensitivity was based on a singular study by Aslaksen et al in 1990 which did not compare ultrasound with CT, but rather with intravenous urography.1,33 Concerns for secondary malignancy risk due to radiation exposure were highlighted by Yecies et al, although risk models secondary to radiation dose should be evaluated in the context of continued evolution in dose reduction techniques.34 In 2020, the AUA acknowledged the very low rates of cancer and the associated costs of imaging evaluation, which led to recommendations for CTU primarily for high-risk groups and thus was more in alignment with other society guidelines.2
3.2.3 MR Urography
MR urography (MRU) offers a similar imaging assessment of the kidneys, urinary collecting system, and bladder compared to CTU without the need for ionizing radiation. Also, MRU can potentially provide more information about lesion characterization than radiography, US, and CT by demonstrating unique tissue properties such as T1/T2 relaxation times and diffusion restriction.23 A typical MRU examination consists of T2-weighted, T1-weighted images with chemical shift imaging and fat suppression before and after gadolinium-based intravenous contrast, as well as diffusion-weighted imaging. With these techniques, both the renal parenchyma and urothelium can be assessed with static T2-weighted (fluid sensitive) sequences and multiphasic post-contrast sequences at predetermined timed intervals, including delayed excretory phases. Although providing diagnostic quality images of the abdomen and pelvis with the full complement of sequences can be technically challenging at times, techniques such as parallel imaging, motion compensation techniques, and compressed sensing can aid in shortening examination times and improve image quality.35 Major limitations to MRU include higher cost, lengthy examination times, limited availability of MR scanners, and reliance on technical expertise.23
Compared to CTU, MRU has similar diagnostic accuracy in evaluating renal masses if protocolled with the full complement of T1 and T2-weighted sequences, chemical shift imaging, diffusion-weighted imaging, and multiphasic post-contrast sequences with the added benefit of potentially differentiating tumor subtypes.35-36 For example, clear cell renal cell carcinoma may show increased T2-weighted signal intensity, intralesional microscopic fat, restricted diffusion, avid enhancement, and washout on dynamic contrast-enhanced images. On the other hand, upper urinary tract UCC may show low signal on T2-weighted images with marked restricted diffusion and progressive low-level enhancement on dynamic contrast-enhanced images. Similar to CTU, excretory phase images on MRU can be evaluated for filling defects that would necessitate direct visualization with cystoscopy or ureteroscopy (Figure 7). Razavi et al have suggested that MRU is less sensitive for detecting UCC compared to CTU with sensitivity and specificity of 69% and 97% versus 96% and 99% respectively.37 Unlike CT, MRU is relatively insensitive for evaluating urinary calculi.35
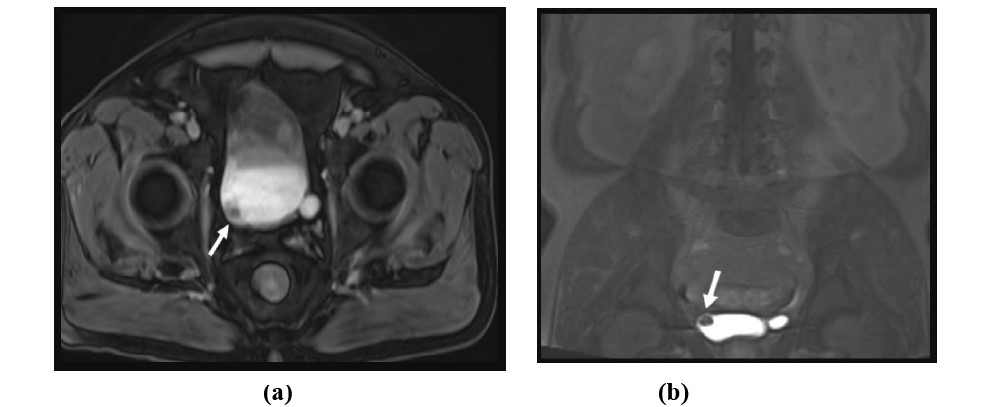
Figure 7: 71-year-old male with gross hematuria. Axial and coronal T1-weighted post-contrast excretory phase MR images with fat saturation demonstrates a small mass in the urinary bladder near the right ureteral orifice consistent with urothelial cell carcinoma.
3.2.4 Renal Ultrasound
Ultrasonography utilizes the piezoelectric effect to produce high-frequency sound waves through a transducer, which interact and reflect off anatomic structures. The reflected sound waves are subsequently returned to the transducer and recorded to produce gray-scale, color Doppler and spectral Doppler images without the need for ionizing radiation. Ultrasonography is safe, portable, and relatively inexpensive compared to cross-sectional techniques such as CT and MRI. The disadvantages of ultrasonography are its inherent operator variability and limitations to optimally visualizing structures secondary to patient body habitus or bowel gas and is particularly limited in directly evaluating the ureters.38
Ultrasonography can effectively evaluate for urinary calculi, which appear as bright, echogenic foci relative to the renal parenchyma, often with a deep hypoechoic shadow due to acoustic attenuation also known as shadowing. Urinary calculi may also demonstrate rapidly alternating colors on color Doppler imaging, also known as a twinkling artifact.39 The pooled sensitivity and specificity for the detection of renal stones on ultrasonography were determined to be 45% and 94% respectively.40 Ultrasonography is generally less sensitive than MDCT and plain radiography for the detection of urinary calculi, particularly for stones <3-4 mm.41-42 However, a combination of abdominal radiography and ultrasound may be a reasonable alternative to CT for the detection of clinically significant stones, while accepting reduced sensitivity for small stones.43
Renal cortical neoplasms such as renal cell carcinoma can have varied appearances on ultrasound although most commonly they appear as a solid or complex cystic cortically-based mass. The echotexture of renal cell carcinoma on ultrasound can be hypoechoic, isoechoic, or hyperechoic relative to the renal parenchyma, which often overlaps with benign lesions such as angiomyolipoma or oncocytoma. Renal cell carcinoma presenting as a cystic mass will demonstrate internal complexity such as mural nodularity and internal septations, which can be readily characterized on ultrasound. Renal cell carcinoma may also demonstrate increased vascularity on color Doppler imaging (Figure 8).44 Contrast-enhanced ultrasound is an emerging technique that utilizes microbubbles as a contrast medium to characterize renal lesions and shows promise in detecting renal malignancy, most notably when examining enhancement characteristics.45-46
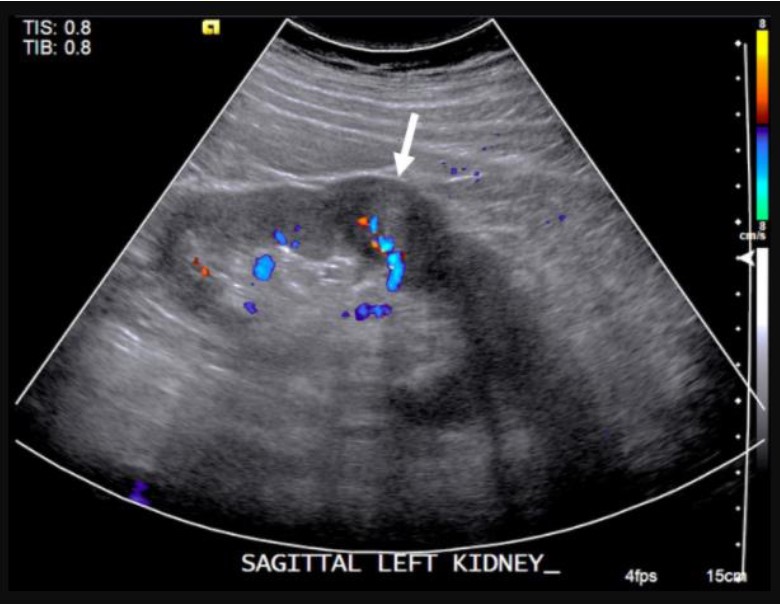
Figure 8: 57-year-old male with incidental left renal mass: Sagittal color Doppler ultrasound of the left kidney demonstrates a partially exophytic mass originating the renal cortex with internal vascularity, consistent with renal cell carcinoma.
Upper urinary tract urothelial cancers such as UCC may present as an echogenic soft tissue mass in the renal sinus (Figure 3).29 The reported sensitivity and specificity in the literature vary widely and are limited by the very low prevalence and heterogeneity of the reference standard. One study of 2138 patients with microhematuria showed ultrasound was 100% sensitive in identifying upper urinary tract malignancy in 12 patients (9 cases of renal cell carcinoma and 3 cases of upper tract urothelial carcinoma); all the patients were followed up clinically with no presenting malignancies within 3 years and only 1.6 cases of upper tract urologic malignancy per 10,000 person-years all found after 3 years.47 Aslaksen et al reported ultrasound detected 3 out of 4 urothelial cancers of the renal pelvis in 1306 patients with tissue diagnosis and clinical follow-up as the reference standard.33 Depending on the degree of infundibular involvement, hydronephrosis may be identified. In contrast to CTU, ultrasonography cannot reliably assess urothelial cancer of the ureters because they are rarely visualized in their entirety due to their retroperitoneal location and overlying bowel gas, although detection of hydronephrosis or even hydroureter may prompt investigation for an obstructing ureteral mass.
3.2.5 Retrograde pyelography
The AUA guidelines mention utilization of retrograde pyelography in conjunction with unenhanced axial imaging and renal ultrasound if there are contraindications to CTU or MRU such as poor renal function or iodinated contrast allergy. Retrograde pyelography involves the opacification of the urinary collecting system with instillation of contrast at the ureteral orifice under fluoroscopic guidance in conjunction with cystoscopy. Retrograde pyelography is a radiographic imaging technique without the cross-sectional information afforded by CT or MRI and therefore is limited by the presence of overlapping structures and reduced soft-tissue contrast. Although the exam may be performed as an office-based procedure, the majority are performed in the operating room during cystoscopy or ureteroscopy which incurs additional cost and need for anesthesia. Retrograde pyelography is also limited by the inability to comprehensively evaluate the renal parenchyma and perirenal soft tissues. Despite these limitations, retrograde pyelography is effective in the evaluation of the urinary tract and ruling out urothelial malignancy. A retrospective study showed retrograde pyelography to be 97% sensitive and 93% specific for urothelial tumors with similar rates found for CTU.48 Lesions concerning for malignancy will demonstrate focal filling defects of the urothelium (Figure 5b). To our knowledge, no studies evaluating for the diagnostic accuracy of retrograde pyelography in microhematuria currently exist.
3.5.6 First-line CTU or Ultrasonography?
The recommendation for CTU versus ultrasound as the initial imaging study for asymptomatic microhematuria is limited by a lack of studies that directly compare diagnostic performance between the two modalities. A recent retrospective study of 575 patients by David et al of a mixed but mainly gross hematuria group of high-risk patients whose ultrasounds were followed by CTU showed the diagnostic accuracy of ultrasound and CTU to be 95.8 and 99.1% respectively (8 renal cell carcinomas and 4 upper tract urothelial carcinomas) with both modalities demonstrating 100% sensitivity.49 This study was strong in comparing both modalities but limited in the rather small number of actual carcinomas. Another recent study this one by Tan et al followed separate groups using either ultrasound or CTU but not both and found that CTU detected three times as many upper urinary tract malignancies (renal cell carcinoma and urothelial carcinoma) than ultrasound: 53/1692 (3.1%) vs 21/2166 (1.0%).10 The role of possible selection bias was not addressed by the authors. To our knowledge, the most numerous renal masses in a head-to-head comparison was performed in 1996 comparing CT with ultrasonography studying 205 masses of which 30 were solid renal cancers. That study demonstrated reduced sensitivity of ultrasound for renal lesions less than 3 cm, although a significant number of lesions smaller than 1 cm were not detected by either modality.50 More studies with greater numbers of cancers using state-of-the-art ultrasound and CTU with attention to diverse populations (i.e. greater BMI) is still needed.
However, given the indolent course of many urologic neoplasms and an increasing elderly patient population detecting smaller cancers may not lead to better life expectancy. More research evaluating the benefit of identifying smaller urologic cancers is warranted, particularly since more small lesions are managed with surveillance or minimally invasive percutaneous techniques rather than surgical resection. Cost and burden on healthcare economics is another important consideration, particularly in the era of healthcare cost containment.51 Halpern et al suggested that replacing ultrasound with CTU in the evaluation of hematuria would increase the cost by $6.5 million per 10000 evaluations to detect just 1 additional cancer.52 Finally, the risk of medical harm from the radiation from a CTU may outweigh any diagnostic benefit especially in low-risk patients that are younger where there are greater radiation risks.53 Long-term effects of radiation exposure by CTU have an attendant potential risk for secondary malignancies, which is likely being mitigated with optimized CTU techniques (e.g. split-bolus technique), iterative reconstruction, and/or dual-energy techniques.1 More large-scale studies will be necessary to further clarify the optimal imaging modality and inform future guidelines.
4. Future Directions
The optimal imaging evaluation for patients with asymptomatic microhematuria is controversial given the high prevalence of hematuria in the general population and low pretest probability for urologic malignancy, particularly in the low-risk population. More large-scale, preferably prospective, studies will be needed to determine optimal image evaluation algorithms that maximize diagnostic yield while minimizing associated health care costs and radiation exposure. Much of the CTU radiation dosing and diagnostic accuracy reflected in the literature are representative of heterogeneous CTU protocols, which continue to evolve and vary by institution. Advances in iterative reconstruction and dual-energy CT will have to be included in the context of radiation dose and diagnostic performance in future studies. Moreover, increased MRI availability and advances in MRU acquisition would warrant further study in the context of hematuria compared to other imaging modalities. Finally, long-term longitudinal data that compares imaging modality with survival and morbidity would be beneficial for evaluating optimal diagnostic approaches.
5. Conclusion and Recommendations
The diagnostic imaging evaluation of hematuria should be tailored to the degree of red blood cells detected by formal urinalysis as well as the clinical evaluation with a thorough history, physical exam and laboratory biomarkers. Patients who meet criteria for gross hematuria should proceed directly to CTU, while patients with microhematuria should be risk stratified according the level of suspicion for urologic malignancy. Generally speaking, low- and intermediate-risk patients should receive ultrasound as the first line upper urinary tract imaging modality with CTU reserved for high-risk patients and to further evaluate patients with abnormal ultrasound findings (Figure 9). MRU or retrograde pyelography may be considered as an alternative for upper urinary tract imaging if there are contraindications for CTU. For low- and intermediate risk patients in particular, shared decision-making between the clinician and the patient regarding the diagnostic evaluation and imaging modality is encouraged. Diagnostic algorithms for microhematuria and the first-line imaging study of choice may vary across international societal guidelines and more research is warranted to identify the imaging modality that optimizes the patient’s pretest probability, diagnostic accuracy, and healthcare cost.
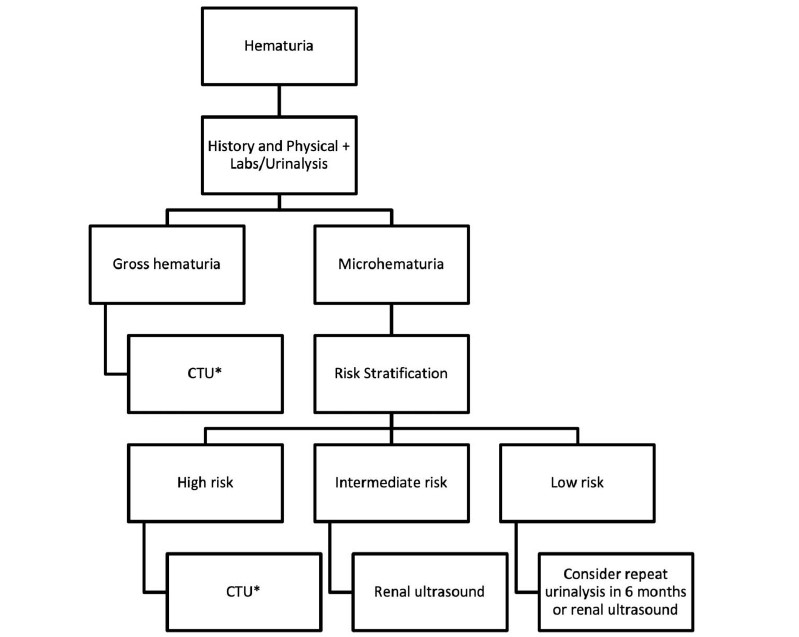
Figure 9: Example diagnostic imaging algorithm for the evaluation of hematuria adopted from the 2020 AUA guidelines. Note. *= MRU or retrograde pyelography may be considered as an alternative to CTU
Disclaimer: The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, Department of Defense or the U.S. Government.
Article Details
The Medical Research Archives grants authors the right to publish and reproduce the unrevised contribution in whole or in part at any time and in any form for any scholarly non-commercial purpose with the condition that all publications of the contribution include a full citation to the journal as published by the Medical Research Archives.
References
2. Barocas DA, Boorjian SA, Alvarez RD, Downs TM, Gross CP, Hamilton BD, Kobashi KC, Lipman RR, Lotan Y, Ng CK, Nielsen ME. Microhematuria: AUA/SUFU guideline. J Urol. 2020;204(4):778-86. https://doi.org/10.1097/JU.0000000000001297
3. Heller MT, Tublin ME. In search of a consensus: evaluation of the patient with hematuria in an era of cost containment. AJR Am J Roentgenol. 2014;202(6):1179-86. https://doi.org/10.2214/AJR.13.12266
4. Woodhouse CR. Symptomless abnormalities. Microscopic haematuria. Br J Hosp Med (Lond). 1982;27(2):163-8.
5. Boman HH, Sten Holmäng H. The results of routine evaluation of adult patients with haematuria analysed according to referral form information with 2-year follow-up. Scand J Urol Nephrol. 2001;35(6):497-501. https://doi.org/10.1080/003655901753367613
6. Edwards TJ, Dickinson AJ, Natale S, Gosling J, McGrath JS. A prospective analysis of the diagnostic yield resulting from the attendance of 4020 patients at a protocol‐driven haematuria clinic. BJU Int. 2006;97(2):301-5. https://doi.org/10.1111/j.1464-410X.2006.05976.x
7. Edwards TJ, Dickinson AJ, Gosling J, McInerney PD, Natale S, McGrath JS. Patient‐specific risk of undetected malignant disease after investigation for haematuria, based on a 4‐year follow‐up. BJU Int. 2011;107(2):247-52. https://doi.org/10.1111/j.1464-410X.2010.09521.x
8. Khadra MH, Pickard RS, Charlton M, Powell PH, Neal DE. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol. 2000;163(2):524-7.
9. Mariani AJ, Mariani MC, Macchioni C, Stams UK, Hariharan A, Moriera A. The significance of adult hematuria: 1,000 hematuria evaluations including a risk-benefit and cost-effectiveness analysis. J Urol. 1989;141(2):350-5. https://doi.org/10.1016/s0022-5347(17)40763-4
10. Tan WS, Sarpong R, Khetrapal P, Rodney S, Mostafid H, Cresswell J, Hicks J, Rane A, Henderson A, Watson D, Cherian J. Can renal and bladder ultrasound replace computerized tomography urogram in patients investigated for microscopic hematuria?. J Urol. 2018;200(5):973-80. https://doi.org/10.1016/j.juro.2018.04.065
11. Linder BJ, Bass EJ, Mostafid H, Boorjian SA. Guideline of guidelines: asymptomatic microscopic haematuria. BJU Int. 2018;121(2):176-83. https://doi.org/10.1111/bju.14016
12. Jung H, Gleason JM, Loo RK, Patel HS, Slezak JM, Jacobsen SJ. Association of hematuria on microscopic urinalysis and risk of urinary tract cancer. J Urol. 2011;185(5):1698-703. https://doi.org/10.1016/j.juro.2010.12.093
13. Froom P, Ribak J, Benbassat J. Significance of microhaematuria in young adults. Br Med J (Clin Res Ed). 1984;288(6410):20-2.
14. Jones DJ, Langstaff RJ, Holt SD, Morgans BT. The value of cystourethroscopy in the Investigation of microscopic haematuria in adult males under 40 years: a prospective study of 100 patients. Br J Urol. 1988;62(6):541-5. https://doi.org/10.1111/j.1464-410x.1988.tb04422.x
15. Mohr DN, Offord KP, Owen RA, Melton LJ. Asymptomatic microhematuria and urologic disease: a population-based study. JAMA. 1986;256(2):224-9. https://doi.org/10.1001/jama.256.2.224
16. Murakami S, Igarashi T, Hara S, Shimazaki J. Strategies for asymptomatic microscopic hematuria: a prospective study of 1,034 patients. J Urol. 1990;144(1):99-101. https://doi.org/10.1016/s0022-5347(17)39379-5
17. Committee on Gynecologic Practice. Committee Opinion No. 703: Asymptomatic Microscopic Hematuria in Women. Obstet Gynecol. 2017;129(6):e168-72. https://doi.org/10.1097/AOG.0000000000002059
18. Lippmann QK, Slezak JM, Menefee SA, Ng CK, Whitcomb EL, Loo RK. Evaluation of microscopic hematuria and risk of urologic cancer in female patients. Am J Obstet Gynecol. 2017;216(2):146-e1. https://doi.org/10.1016/j.ajog.2016.10.008
19. Dahlman P, van der Molen AJ, Magnusson M, Magnusson A. How much dose can be saved in three-phase CT urography? A combination of normal-dose corticomedullary phase with low-dose unenhanced and excretory phases. AJR Am J Roentgenol. 2012;199(4):852-60. https://doi.org/10.2214/AJR.11.7209
20. Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Cowan NC, Dominguez-Escrig JL, Gontero P, Mostafid AH, Palou J. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol. 2020;79(1):62–79. https://doi.org/10.1016/j.eururo.2020.05.042
21. Wolfman DJ, Marko J, Nikolaidis P, Khatri G, Dogra VS, Ganeshan D, Goldfarb S, Gore JL, Gupta RT, Heilbrun ME, Lyshchik A. ACR appropriateness criteria® hematuria. J Am Coll Radiol. 2020;17(5):S138-47. https://doi.org/10.1016/j.jacr.2020.01.028
22. Potenta SE, D’Agostino R, Sternberg KM, Tatsumi K, Perusse K. CT urography for evaluation of the ureter. Radiographics. 2015;35(3):709-26. https://doi.org/10.1148/rg.2015140209
23. Silverman SG, Leyendecker JR, Amis Jr ES. What is the current role of CT urography and MR urography in the evaluation of the urinary tract?. Radiology. 2009;250(2):309-23. https://doi.org/10.1148/radiol.2502080534
24. Kim SH, Kim MJ, Lee HJ, Cho SH. Comparison of full-and half-dose image reconstruction with filtered back projection or sinogram-affirmed iterative reconstruction in dual-source single-energy MDCT urography. AJR Am J Roentgenol. 2018;211(3):641-8. https://doi.org/10.2214/AJR.17.19370
25. Kaza RK, Platt JF, Cohan RH, Caoili EM, Al-Hawary MM, Wasnik A. Dual-energy CT with single-and dual-source scanners: current applications in evaluating the genitourinary tract. Radiographics. 2012;32(2):353-69. https://doi.org/10.1148/rg.322115065
26. Kang SK, Huang WC, Pandharipande PV, Chandarana H. Solid renal masses: what the numbers tell us. AJR Am J Roentgenol. 2014;202(6):1196-206. https://doi.org/10.2214/AJR.14.12502
27. Chlapoutakis K, Theocharopoulos N, Yarmenitis S, Damilakis J. Performance of computed tomographic urography in diagnosis of upper urinary tract urothelial carcinoma, in patients presenting with hematuria: systematic review and meta-analysis. Eur J Radiol. 2010;73(2):334-8. https://doi.org/10.1016/j.ejrad.2008.10.026
28. Dyer R, DiSantis DJ, McClennan BL. Simplified imaging approach for evaluation of the solid renal mass in adults. Radiology. 2008;247(2):331-43. https://doi.org/10.1148/radiol.2472061846
29. Browne RF, Meehan CP, Colville J, Power R, Torreggiani WC. Transitional cell carcinoma of the upper urinary tract: spectrum of imaging findings. Radiographics. 2005;25(6):1609-27. https://doi.org/10.1148/rg.256045517
30. Trinh TW, Glazer DI, Sadow CA, Sahni VA, Geller NL, Silverman SG. Bladder cancer diagnosis with CT urography: test characteristics and reasons for false-positive and false-negative results. Abdom Radiol (NY). 2018;43(3):663-71. https://doi.org/10.1007/s00261-017-1249-6
31. Davis R, Jones JS, Barocas DA, Castle EP, Lang EK, Leveillee RJ, Messing EM, Miller SD, Peterson AC, Turk TM, Weitzel W. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol. 2012;188(6S):2473-81. https://doi.org/10.1016/j.juro.2012.09.078
32. Lisanti CJ, Toffoli TJ, Stringer MT, DeWitt RM, Schwope RB. CT evaluation of the upper urinary tract in adults younger than 50 years with asymptomatic microscopic hematuria: is IV contrast enhancement needed? AJR Am J Roentgenol. 2014;203(3):615-9. https://doi.org/10.2214/AJR.13.11891
33. Aslaksen A, Halvorsen OJ, Göthlin JH. Detection of renal and renal pelvic tumours with urography and ultrasonography. Eur J Rad. 1990;11(1):54-8. https://doi.org/10.1016/0720-048x(90)90103-i
34. Yecies T, Bandari J, Fam M, Macleod L, Jacobs B, Davies B. Risk of radiation from computerized tomography urography in the evaluation of asymptomatic microscopic hematuria. J Urol. 2018;200(5):967-72. https://doi.org/10.1016/j.juro.2018.05.118
35. Abreu-Gomez J, Udare A, Shanbhogue KP, Schieda N. Update on MR urography (MRU): technique and clinical applications. Abdom Radiol (NY). 2019;44(12):3800-10. https://doi.org/10.1007/s00261-019-02085-1
36. Lopes Vendrami C, Parada Villavicencio C, DeJulio TJ, Chatterjee A, Casalino DD, Horowitz JM, Oberlin DT, Yang GY, Nikolaidis P, Miller FH. Differentiation of solid renal tumors with multiparametric MR imaging. Radiographics. 2017;37(7):2026-42. https://doi.org/10.1148/rg.2017170039
37. Razavi SA, Sadigh G, Kelly AM, Cronin P. Comparative effectiveness of imaging modalities for the diagnosis of upper and lower urinary tract malignancy: a critically appraised topic. Acad Radiol. 2012;19(9):1134-40. https://doi.org/10.1016/j.acra.2012.05.004
38. O'Connor OJ, McSweeney SE, Maher MM. Imaging of hematuria. Radiol Clin North Am. 2008;46(1):113-32. https://doi.org/10.1016/j.rcl.2008.01.007
39. Dillman JR, Kappil M, Weadock WJ, Rubin JM, Platt JF, DiPietro MA, Bude RO. Sonographic twinkling artifact for renal calculus detection: correlation with CT. Radiology. 2011;259(3):911-6. https://doi.org/10.1148/radiol.11102128
40. Ray AA, Ghiculete D, Pace KT, Honey RJ. Limitations to ultrasound in the detection and measurement of urinary tract calculi. Urology. 2010;76(2):295-300. https://doi.org/10.1016/j.urology.2009.12.015
41. Fowler KA, Locken JA, Duchesne JH, Williamson MR. US for detecting renal calculi with nonenhanced CT as a reference standard. Radiology. 2002;222(1):109-13. https://doi.org/10.1148/radiol.2221010453
42. Gokce MI, Ozden E, Suer E, Gulpinar B, Gulpınar O, Tangal S. Comparison of imaging modalities for detection of residual fragments and prediction of stone related events following percutaneous nephrolitotomy. Int Braz J Urol. 2015;41(1):86-90. https://doi.org/10.1590/S1677-5538.IBJU.2015.01.12
43. Coursey CA, Casalino DD, Remer EM, Arellano RS, Bishoff JT, Dighe M, Fulgham P, Goldfarb S, Israel GM, Lazarus E, Leyendecker JR. ACR appropriateness criteria® acute onset flank pain–suspicion of stone disease. Ultrasound Q. 2012;28(3):227-33. https://doi.org/10.1097/RUQ.0b013e3182625974
44. Jinzaki M, Matsumoto K, Kikuchi E, Sato K, Horiguchi Y, Nishiwaki Y, Silverman SG. Comparison of CT urography and excretory urography in the detection and localization of urothelial carcinoma of the upper urinary tract. AJR Am J Roentgenol. 2011;196(5):1102-9. https://doi.org/10.2214/AJR.10.5249
45. Atri M, Tabatabaeifar L, Jang HJ, Finelli A, Moshonov H, Jewett M. Accuracy of contrast-enhanced US for differentiating benign from malignant solid small renal masses. Radiology. 2015;276(3):900-8. https://doi.org/10.1148/radiol.2015140907
46. Lu Q, Huang BJ, Xue LY, Fan PL, Wang WP. Differentiation of renal tumor histotypes: usefulness of quantitative analysis of contrast-enhanced ultrasound. AJR Am J Roentgenol. 2015;205(3):W335-42. https://doi.org/10.2214/AJR.14.14204
47. Smith MR, Read KC, Stegman ML, Kroll NJ, Van Every MJ. Evaluation of asymptomatic microscopic hematuria by renal ultrasound to detect upper tract malignancy: a 20-year experience in a community hospital. Urology. 2019;133:34-9. https://doi.org/10.1016/j.urology.2019.07.009
48. Cowan NC, Turney BW, Taylor NJ, McCarthy CL, Crew JP. Multidetector computed tomography urography for diagnosing upper urinary tract urothelial tumour. BJU Int. 2007;99(6):1363-70. https://doi.org/10.1111/j.1464-410X.2007.06766.x
49. David RA, James B, Adeloye D, Bose P, Rai B, KandaSwamy GV. Accuracy of ultrasound vs computed tomography scan for upper urinary tract malignancies and development of a risk-based diagnostic algorithm for haematuria in a UK tertiary centre. Int Urol Nephrol. 2021;53(1):49-57. https://doi.org/10.1007/s11255-020-02615-7
50. Jamis-Dow CA, Choyke PL, Jennings SB, Linehan WM, Thakore KN, Walther MM. Small (< or= 3-cm) renal masses: detection with CT versus US and pathologic correlation. Radiology. 1996;198(3):785-8. https://doi.org/10.1148/radiology.198.3.8628872
51. Subak LL, Grady D. Asymptomatic microscopic hematuria—rethinking the diagnostic algorithm. JAMA Intern Med. 2017;177(6):808-9. https://doi.org/10.1001/jamainternmed.2017.0758
52. Halpern JA, Chughtai B, Ghomrawi H. Cost-effectiveness of common diagnostic approaches for evaluation of asymptomatic microscopic hematuria. JAMA Intern Med. 2017;177(6):800-7. https://doi.org/10.1001/jamainternmed.2017.0739
53. Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Craft AW, Parker L. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499-505. https://doi.org/10.1016/S0140-6736(12)60815-0