Exposure to Antibodies Anti-Chlamydophila Pneumoniae Associated to Respiratory Symptoms of Asthma among Adolescents.
Main Article Content
Abstract
Previous report shows relationship between C. pneumonie and Asthma. In this study, we assessed the association between exposure to C. pneumoniae infection and the risk of bronchial asthma in adolescents in the State of Morelos, Mexico. An analysis was performed on a population-based cohort study of 80 adolescents with respiratory symptoms of asthma defined by ISAAC and 202 healthy adolescents between 12 and 17 years old. The information was collected twice from questionnaires, anthropometry, and sampling. Excessive weight gain was determined by calculating body mass index, and exposure to the specific antibodies IgM, IgG, and IgA was detected by microimmunofluorescence. The geometric means were calculated for titers of C. pneumoniae. The odds ratio was used across multiple models. The results showed that the exposure to C. pneumoniae was very high in the study population (67.2%). All immunoglobulins were significantly increased in patients with asthma symptoms compared with the healthy population (17% for IgG, 34% for IgM, and 52% for IgA). In multiple models, the IgM and IgA immunoglobulins were found to be associated with asthma (OR, 2.4; 95% CI, 1.4–4.2 and OR, 2.4; 95% CI, 1.2–4.8, respectively). Our study reflects a high seroprevalence of C. pneumoniae in the population; this seroprevalence is higher in young people with asthma. Specific immunoglobulins to C. pneumoniae are associated with IgM and IgA. The epidemiological significance of our results influences the timely monitoring and management of infections acquired at an early age that persist, or recur, for much of the juvenile life. Additional studies are needed to validate our findings.

1. Introduction
Asthma is a chronic inflammatory disease that is related to hyper-reactivity of the airways and characterized by repeated episodes of wheezing and dyspnea. This disease represents the most frequent chronic respiratory disease among adolescents1 in different countries, with prevalence ranging from 2.1% to 32.2% in children 12 to 14 years old.2 For example, Brazil has reported a prevalence of between 12.4 to 25.2% in adolescents 13–14 years old,3,4 while the estimated prevalence in Mexico City is 9.9% in the same age group5 and approximately 5% in all adolescents. In other locations in Mexico, the prevalence are similar, including 12% in Merida and Yucatan and 7.3% in Tamaulipas.6, 7
Epidemiological and clinical studies have suggested that there is a causal relationship between recurrent infections of the upper respiratory tract and the development of airway hyper-reactivity and respiratory allergies in children during subsequent subclinical or asymptomatic infection.8-10 Chlamydophila pneumoniae (C. pneumoniae) has been reported since the early 1990's to have a significant etiopathogenic role in the development of acute, atypical infections of the respiratory tract. C. pneumoniae infection is potentially associated with the onset of wheezing and asthma and with a greater exacerbation of the symptomatology of respiratory diseases, including pneumonia, chronic obstructive pulmonary disease, and chronic asthma.11, 12
There is evidence that a wide variety of microorganisms are present in the lower airway and may play a role in asthma pathogenesis, suggests that manipulating the airway microbiome may be a novel approach towards this goal. Studies confirm the existence of an infectious etiology mediated by C. pneumoniae.13,14 Among the various infections associated with asthma, this obligate intracellular respiratory pathogen C. pneumoniae is of particular interest, as it is associated with both asthma severity and treatment resistance.15,16,17 Likewise, evidence links C. pneumoniae infection with both de novo asthma (asthma onset during/after an acute lower respiratory tract infection in a previously non-asthmatic individual, also referred to as the “infectious asthma” syndrome). 18, 19
Has been shown by serology to be one of the most prevalent infectious agents worldwide.20
Recently, the seroprevalence of the infection in asthmatics and the frequency of associated exacerbations has varied as a function of either the populations studied or the geographic area; for example, positive serology for C. pneumoniae of up to 48.1% has been reported in children and adolescents in Peru. 21
Fetal exposure to tobacco smoke has been given special consideration. It has been demonstrated that the newborns of mothers who smoke have poorer respiratory function and a higher prevalence of respiratory disease or wheezing. 22 Another factor reported for the deterioration of pulmonary function is obesity. 23 Mexico has one of the leading places in the world, both in adulthood (with a prevalence of 71%) as well as in childhood and adolescence (28% in males and 30% in females). 24
With this background, we proposed to evaluate the possible association between exposure to C. pneumoniae and the risk of asthma in adolescents in Mexico.
2. Materials and methods
2.1 Study population
The information was obtained from a cohort study, which recruited 13,293 students of both genders, aged between 11 to 17 years old, from the public education system of the State of Morelos in 1998–2001. 25 The participants were selected during the baseline measurements by random sampling stratified by geographical area of the state and by clusters of the lists that integrated the public schools of the State of Morelos. The project was accepted by the State Institute of Public Education] and approved by the Ethics Committee of the National Institute of Public Health. The participants signed letters of agreement, and consent was given by the parents. In our analysis, a total of 282 adolescents were selected from the original list of the cohort, which consisted of the complete information from the questionnaires and blood samples.
2.2 Variable results
We used the standard definition of asthma proposed by the International Study of Asthma and Allergies in Childhood (ISAAC).26 Briefly, the asthma variable was established under the following conditions: If the diagnosis of asthma or asthmatic bronchitis was established by a treating physician, or if the adolescent had a dry cough and/or repetitive wheezing nightly or after the performance of exercise in the last 12 months.
2.3 Definition of exposure
Serologic tests were performed in a blinded fashion using the microimmunofluorescence (MIF) test developed by Wang and Grayston.27 C. pneumoniae elementary bodies were used as antigens in the MIF test, and the IgG, IgM, and IgA were detected. C. trachomatis and C. psittaci antigens were included in the test. Cross-reactions between the different chlamydial species and the immunological activity of the LPS in the C. pneumoniae and C. trachomatis antigens appear to be low. In the literature, IgA has been considered to be a marker of chronic C. trachomatis infections.28
2.4 Test Principle: This test was based on the indirect detection of the IgG, IgM, and IgA antibodies against C. pneumoniae using fluorescein isothiocyanate (FITC) as the marker compound21. The antibodies for C. pneumoniae in the patient’s serum were combined with C. pneumoniae antigens fixed on the surface of a glass slide. The slide was washed, followed by the addition of fluorescein conjugated with anti-human antibodies. The slides were then revealed and detected with an epifluorescence microscope.i An acute infection was defined in a patient as an elevated IgG titer 4 times higher relative to standard sera; if two paired sera were unavailable, an IgM titer of 16 or a single IgG titer of 512 with a compatible clinical history were usually considered sufficient. A past infection was defined as IgG titers of 16 but 512, whereas a recurrent infection was defined as IgA titers of 16. The MIF method, with a high negative predictive value (98%) and a high sensitivity for the detection of chlamydial infections, was used.
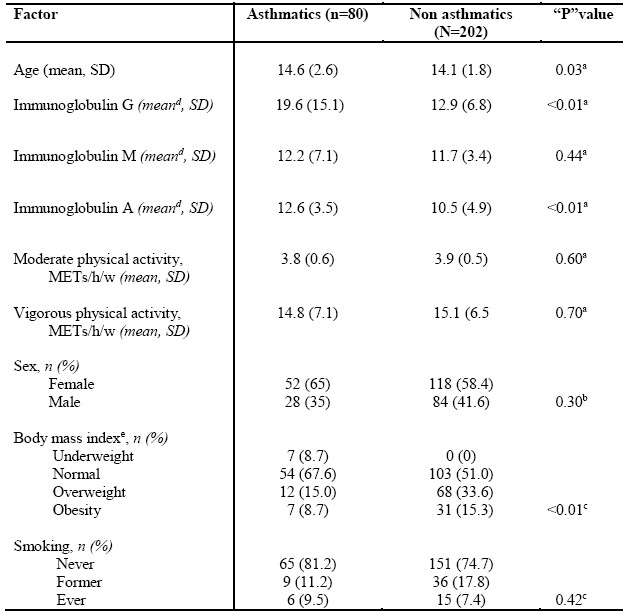
Table 1. Baseline characteristics of population (n=282). Mexico, 2012
2.5 Co-variables
The body composition information was obtained via standard measurement procedures. Briefly, a Tanita digital scale with a precision of 0.01 mm of error was used to measure weight; a stadiometer was used to measure height. The students were measured and weighed barefoot and with minimal clothing. The body mass index (BMI) was calculated according to formula: weight (kg) / height (m2). Physical activity was measured continuously in Metabolic Equivalent for Task (MET) units through a questionnaire of daily physical activity previously validated for the Mexican population. Moderate physical activity was identified based on a cut-off point of 3 to 5 METs/hour/week; vigorous physical activity was defined by a cutoff point of > 5 METs/hour/week. The smoking variable was evaluated in 3 categories according to each students’ status as a non-smoker, ex-smoker, and current smoker.
2.6 Statistical analysis
The results of the descriptive analysis are presented as percentages, means, and standard deviations. Student’s t-tests were used to compare two samples of continuous variables, whereas 2-tailed ANOVA tests were used for three samples. For this analysis, we used the inverse of the dilution results of the MIF reading (converted into the base 2 logarithmic scale) and reported the resultant geometric means. Finally, a multiple logistic regression model was used, and the 95% confidence intervals were calculated to determine statistical significance.
3. Results
In the baseline evaluation, the asthmatic participants were slightly older than the controls (p = 0.03); twice as many control subjects were overweight or obese compared to the asthma patients (p < 0.01). Variables such as sex, smoking, and physical activity showed no differences between the two groups. The asthmatic participants showed higher IgG and IgA concentrations compared with the controls (p < 0.01) (Table1).
When comparing the concentrations of the immunoglobulins during the follow-up, we found a negative absolute difference in IgM and IgA but not in IgG, in the asthmatic participants. The controls showed negative absolute differences in the concentrations of IgM and IgA that were similar to the cases, whereas IgG showed a positive absolute difference. The frequency of exposure during the period was 55% for IgG in the asthmatic participants, whereas in the controls, it was 45.5%. The exposure to IgM in the cases was 50.6% compared to the controls, which showed only 33.6%. The frequency of exposure for IgA decreased, but in the asthmatic participants, it was double when compared with the controls (23.1 vs. 11.1%, respectively) (Table 2). This result indicated that the asthmatic participants showed increases in the exposure of IgG, IgM, and IgA of 17%, 34%, and 52%, respectively. The overall prevalence of all the immunoglobulins during the study period was 67.2% (77.5% for the cases and 59.4% for the controls).
Table 2. Comparison of Immunoglobulin levels between groups in baseline and follow-up (n=282), Mexico 2012
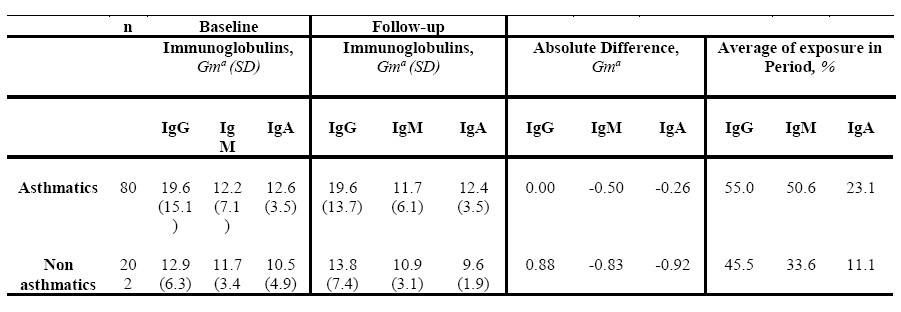
a Geometric mean
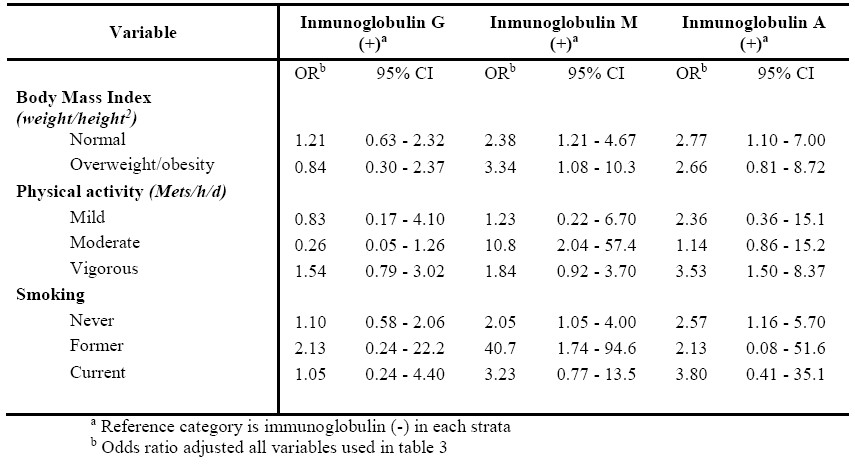
In the multiple regression models, IgG showed no association (OR, 1.1; 95% CI, 0.6–1.9), whereas IgM (OR, 2.4; 95% CI, 1.4–4.2) and IgA (OR, 2.4; 95% CI, 1.2–4.8) were correlated with an increased risk of asthma (Table 3). We stratified by BMI, physical activity and tobacco use founded an association between IgM (OR, 2.38; 95%CI, 1.21-4.67) and IgA (OR, 2.77; 95%CI, 1.10-7.00) to obesity and normal weight respectively, meanwhile IgM was associated to moderate physical activity. On the other hand never smokers were associated to IgM (OR, 2.05; 95%CI, 1.05-4.00) and to IgA (OR, 2.57; 95%CI, 1.16-5.70) (Table 4).
Table 4. Odds ratio and confident intervals of asthma cases by immunoglobulin levels stratified for BMI, physical activity and tobacco use. Mexico, 2012.
4. Discussion
4.1 Seroprevalence
The selected population showed a C. pneumoniae infection rate of 67.2% according to microimmunofluorescence. However, the external validity of our study is limited to adolescents in the State of Morelos.
The prevalence of infection was notably higher in the asthmatic cases compared with the healthy population. When evaluating the exposure according to the type of immunoglobulin, it was found that IgG was the most prevalent, followed by IgM and IgA. Notably, the largest difference in exposure to C. pneumoniae was correlated with IgA in the asthmatic cases when compared with the healthy controls. These results are comparable with the findings of other international groups, which have reported different prevalences of anti-C. Pneumoniae detected by MIF. For example, in Middle Eastern countries such as Israel,29 seroprevalences of 31% and 74% have been found in children and in adults, respectively, with no seasonal preferences; by contrast, in Jordan,30 the overall seropositivity was 54%, which increased with increasing age, and only 14.7% of cases of infection, as indicated by IgM, were recently acquired. On the other hand, in Iran, 31 11.6% and 65.5% seroprevalences for IgG in children and adults, respectively, have been detected by ELISA. In Singapore, 32 the seroprevalence for C. pneumoniae (IgG) determined by MIF reached up to 70%; the seroprevalence was the lowest in the younger age group (18–29 years), 48% for both sexes, and ascended with age up to 78.9%. That study population included Chinese, Malays, and Asian Indians, who showed no differences when compared.
4.2 Associated immunoglobulins
In our study, multiple models showed a significant 2-fold association with IgM and IgA (OR, 2.4) in subjects with asthma, whereas IgG showed no association (95% CI, 0.6–1.9). Previous reports have shown diversity in relation to these immunoglobulins. Thus, a case-control study conducted with 196 children and young individuals showed no association with either IgG (p = 0.12) or IgA (p = 0.18) detected via MIF. 33 However, in a cross-sectional study of 369 young adults, C. pneumoniae infection measured by means of IgG and IgM (MIF) showed an association with respiratory symptoms of cough and phlegm in asthmatic subjects (OR, 1.80; 95% CI: 1.01–3.36 and OR 2.31; 95% CI: 1.20–4.42, respectively). 34 Another study was conducted on 3 groups of subjects: 141 asthmatic participants, 62 allergic participants without asthma, and 125 healthy controls were evaluated, and a higher prevalence for IgA (OR, 5.9; 95% CI, 1.7–26.2) and IgA+IgG (OR, 5.2; 95% CI, 1.6–25.8) were found in the allergic and asthmatic subjects compared to the non-asthmatics. 35 On the other hand, in a study aimed at determining whether the prevalence changes with seasonality, 127 asthmatic participants were recruited and were compared with 391 controls from young adults. The authors found a decrease in the seroprevalence of IgG in both groups at 6 months of observation. This number was reduced in the summer by more than 20% in both groups, but increased in the winter by 10% in the asthmatic participants and by 5% in the non-asthmatic participants. 36 We also performed a cohort analysis using absolute values and a period seroprevalence, which showed an increase of all the immunoglobulins, with IgA being the most prominent and presenting with average values that were doubled in the asthmatic participants. However, seasonality was not assessed in the study periods.
Several reports have been showed obesity related to increased risk of asthma among children and teenagers using clinical criteria, for instance a cohort study37 among 2,171 children and adolescents in USA founded a positive association (HR,1.51; 95% CI, 1.08-2.10 ). In the same way in Greece38, a case-control study performed among 514 adolescents reported an association between obesity and asthma (OR, 1.52, 95% CI 1.03-2.70). In the USA it was reported that the adjusted risk for incident asthma was increased among children who were overweight (relative risk [RR]: 1.17; 95% confidence interval (CI): 1.10–1.25) and obese (RR: 1.26; 95% CI: 1.18–1.34). 39 In China40 a meta-analysis was done, a total of 13 studies were included, including 2 case–control studies, 6 cohort studies, and 5 cross-sectional studies. It was observed a positive association between abdominal obesity and asthma (OR = 1.47, 95% CI 1.35–1.59). Likewise, in China 41 was found in a meta-analysis, a bidirectional association between obesity and asthma
during childhood and adolescence. There was a statistically significant association between obesity and increased risk of physician-diagnosed asthma in children and adolescents (RR was 1.39 (95% CI: 1.28, 1.50; p < 0.001), For the association of asthma with risk of childhood obesity, (RR was 1.47 (95%CI: 1.25, 1.72; p < 0.001). Another cohort study in USA42 showed direct association between persistent obesity and asthma diagnosed by clinical criteria and allergen specific IgE levels (RR, 2.4; 95% CI, 1.2-4.7). The National Health and Nutrition Examination Survey (NHANES) using data from 1999-2006 reported an association between obesity and asthma defined by clinical criteria, self-report and stratified by IgE (OR, 2.46, 95% CI, 1.21-5.02 for atopic children and OR, 1.34, 95% CI, 0.70-2.57 for non-atopic children). 43 Our study showed consistency with these reports especially in IgM (OR, 3.34; 95% CI, 1.08-10.3).
Participation of physical activity as risk factor asthma has been reported for few authors, but most important issue is referent to increased physical activity to prevent asthma or asthma crisis. Our results shoed and direct association to moderate physical activity, nevertheless prevalence in both (asthmatics and non-asthmatics) was similar and the sample size was small.
4.3 Limitations
Although some our results are consistent with different reports, we must note that our study was conducted with a reduced sample size despite being embedded in a cohort study.
We considered the IgG titers to indicate chronic exposure and those of IgA to indicate re-infection; however, this should be taken with caution because, due to the immune response, we have not necessarily affirmed persistent infection.44 However, our cohort design and the high prevalence of C. pneumoniae enabled us to show that the infection is chronic and is consistent with the persistence of the symptoms in the participants. Another potential limitation is that we did not evaluate IgE, which has been reported by recent studies of C. pneumoniae infection in asthmatics be an important immunoglobulin, reaching a seroprevalence of up to 50% in these patients when compared to the healthy population. 16 The proposed mechanism responsible for this difference is the capability of these immunoglobulins to induce an allergic response by producing T-helper 2 peripheral blood mononuclear cells (PBMC).45 On the other hand, there may be an association between C. pneumoniae, asthma and production of IgE responses in PBMC; IL-4 is required for IgE production. 46
5. Conclusions
Our study reflects a high seroprevalence of C. pneumoniae in the population that is higher in young people with symptoms of asthma. Similarly, IgM and IgA are the specific immunoglobulins associated with C. pneumoniae infection. The epidemiological significance of our results influences the timely monitoring and management of C. pneumoniae infection in populations exposed at an early age to this bacterium and in cases that persist or recur throughout much of the juvenile life.
More studies are needed to validate our findings and to evaluate bronchial reactivity to C. pneumoniae.
Acknowledgements
This work was carried out with funds granted by the Bristol-Mayers Squibb Foundation (New-York), under initiative called “Better Health for Women: A Global Health Program”. Likewise, National Council of Science and Technology of Mexico (CONACyT in Spanish) number: 34487-M.
Authorship
GGE was the principal investigator for laboratory work and assisted with data interpretation and manuscript writing; MMG, GRP, and LMSZ were responsible for the biochemical data collection and analysis, and provided critical review of the manuscript; ELP contributed to data interpretation and provided critical review of the manuscript; ESM was responsible for data analyses, data interpretation and primary manuscript writing. All authors have read and approved the final manuscript.
Ethical responsibilities
Protection of people
The authors declare that the procedures followed were in accordance with the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
Rigth to privacy and informed consent. The participants signed letters of agreement, and consent was given by the parents.
Conflict of interests
The authors declare that they have no conflict of interest.
Article Details
The Medical Research Archives grants authors the right to publish and reproduce the unrevised contribution in whole or in part at any time and in any form for any scholarly non-commercial purpose with the condition that all publications of the contribution include a full citation to the journal as published by the Medical Research Archives.
References
2. Laforest L, Yin D, Kocevar VS, Pacheco Y, Dickson N, Gormand F et al. Association between asthma control in children and loss of workdays by caregivers. Allergy Asthma Immunol. 2004; 93:265-71. doi: 10.1016/s1081-1206(10)61499-8
3. Gomes de Luna M de F, Gomes de Luna JR, Fisher GB, de Almeida PC, Chiesa D, Gurgel M et al. Factors associated with asthma in adolescents in the city of Fortaleza, Brazil. J Asthma. 2015; 52:485-91. doi: 10.3109/02770903.2014.984841
4. Barreto ML, Ribeiro-Silva Rde C, Malta DC, Oliveira-Campos M, Andreazzi MA, Cruz AA. Prevalence of asthma symptoms among adolescents in Brazil: National Adolescent School-based Health Survey (PeNSE 2012). Rev Bras Epidemiol. 2014; 17 Suppl 1:106-15. doi:
10.1590/1809-4503201400050009
5. Del-Rio-Navarro B, Del Rio-Chivardi JM, Berber A, Sienra-Monge JJ, Rosas-Vargas MA, Baeza-Bacab M. Asthma prevalence in children living in north Mexico City and a comparison with other Latin American cities and world regions. Allergy Asthma Proc. 2006; 27:334-40. doi: 10.2500/aap.2006.27.2880
6. Vargas Becerra MH. [Epidemiology of asthma]. Rev Alerg Mex. 2009; 56 Suppl 1:S3-9.
7. Torres Ferman IA, Vazquez Nava F, Calafell Ceballos RA, Vázquez Rodríguez EM, Almeida Arvizu VM, Barrientos Gómez MC, et al. [Asthma prevalence in adolescents. Relation to sex and active smoking]. Rev Alerg Mex. 2010; 57:146-152.
8. Fundora-Hernández H, Venero-Fernández SJ, Suárez-Medina R, Mora-Faife EL, García-García G, del Valle-Infante I et al. What are the main environmental exposures associated with elevated IgE in Cuban infants? A population-based study. Trop Med Int Health. 2014; 19:545-554. doi: 10.1111/tmi.12293
9. Guilbert TW, Denlinger LC. Role of infection in the development and exacerbation of asthma. Expert Rev Respir Med. 2010; 4:71-83. doi: 10.1586/ers.09.60
10. Patel KK, Anderson E, Salva PS, Webley WC. The prevalence and identity of Chlamydia-specific IgE in children with asthma and other chronic respiratory symptoms. Respir Res. 2012; 13:32. doi: 10.1186/1465-9921-13-32
11. Allegra L, Blasi F, Centanni S, Cosentini R, Denti F, Raccanelli R et al. Acute exacerbations of asthma in adults: role of Chlamydia pneumoniae infection. Eur Respir J. 1994; 7:2165-2168. doi: 10.1183/09031936.94.07122165
12. Hahn DL, Dodge RW, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA. 1991; 266:225-230.
13. Webley WC, Salva PS, Andrzejewski C, Cirino F, West CA, Tilahun et al. The bronchial lavage of pediatric patients with asthma contains infectious Chlamydia. Am J Respir Crit Care Med. 2005; 171:1083-88. doi: 10.1164/rccm.200407-917OC
14. Webley WC , Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017; 18:98.doi: 10.1186/s12931-017-0584-z.
15. Cho Y, Kim T, Lee T, Moon K, Lee J, Kim Y et al. Chlamydia pneumoniae infection enhances cellular proliferation and reduces steroid responsiveness of human peripheral blood mononuclear cells via a tumor necrosis factor-alpha-dependent pathway. Clin Exp Allergy. 2005; 35:16251631. doi:10.1111/j.1365-2222.2005.02391.x
16. Hahn DL, Schure A, Patel K, Childs T, Drizik E, Webley W. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012; 7:e35945. doi:10.31371/journal.pone.0035945
17. Hahn DL, Grasmick M, Hetzel S, Yale S. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012; 25:442–59. doi: 10.3122/jabfm.2012.04.110309
18. von Hertzen LC. Role of persistent infection in the control and severity of asthma: focus on Chlamydia pneumoniae. Eur Respir J. 2002; 19:546–556. doi: 10.1183/09031936.02.00254402
19. von Hertzen L, Vasankari T, Liippo K, Wahlström E, Puolakkainen M. Chlamydiapneumoniae and severity of asthma. Scand J Infect Dis. 2002; 34:22–7. doi: 10.1080/00365540110077155
20. Cook PJ, Davies P, Tunnicliffe W, Ayres JG, Honeybourne D, Wise R. Chlamydia pneumoniae and asthma. Thorax.1998; 53:254-259.doi: 10.1136/thx.53.4.254
21. Miranda Candelario JF. Atypical microorganisms in children whit community adquired pneumonia: EsSalud Grau Emergency Hospital - Period 2008. Acta Méd Peruana. 2012; 29:17-22.
22. Cabrera Navarro P, Caminero Luna J. [Risk factors in asthma]. Arch Bronconeumol. 2001; 37:248-256. doi: 10.1016/s0300-2896(01)75062-4
23. Del-Rio-Navarro BE, Castro-Rodriguez JA, Garibay Nieto N, Berber A, Toussaint G, Sienra-Monge JJ et al. Higher metabolic syndrome in obese asthmatic compared to obese nonasthmatic adolescent males. J Asthma. 2010; 47:501-506. doi: 10.3109/02770901003702808
24. Barquera S, Campos-Nonato I, Hernandez-Barrera L, Pedroza A, Rivera-Dommarco JA. [Prevalence of obesity in Mexican adults 2000-2012]. Salud Publica Mex. 2013; 55 Suppl 2:S151-160.
25. Lazcano-Ponce EC, Hernandez B, Cruz-Valdez A, Allen B, Díaz R, Hernández C et al. Chronic disease risk factors among healthy adolescents attending public schools in the state of Morelos, Mexico. Arch Med Res. 2003; 34:222-236. doi: 10.1016/S0188-4409(03)00042-0
26. Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483-491. doi: 10.1183/09031936.95.08030483
27. Wang SP, Kuo CC, Grayston JT. Formalinized Chlamydia trachomatis organisms as antigen in the micro-immunofluorescence test. J Clin Microbiol. 1979;10:259-261. doi: 10.1128/JCM.10.2.259-261.1979
28. Chaim W, Sarov B, Sarov I, Piura B, Cohen A, Insler V. Serum IgG and IgA antibodies to Chlamydia in ectopic pregnancies. Contraception. 1989;40:59-71. doi: 10.1016/0010-7824(89)90028-0
29. Ben-Yaakov M, Eshel G, Zaksonski L, Lazarovich Z, Boldur I. Prevalence of antibodies to Chlamydia pneumoniae in an Israeli population without clinical evidence of respiratory infection. J Clin Pathol. 2002;55:355-358. doi: 10.1136/jcp.55.5.355
30. Al-Younes HM. High prevalence of Chlamydia pneumoniae infection in an asymptomatic Jordanian population. J Microbiol Immunol Infect. 2014;47:412-417. doi: 10.1016/j.jmii.2013.04.004
31. Nemati M, Mirzaee V, Shaabani Z, Zarrin M, Mirabdolahi SA, Bagheri-Jamebozorgi M, et al. Specific serum immunoglobulin G against Chlamydia pneumoniae in helthy children and adults in south-east of IRAN. J Ayub Med Coll Abbottabad. 2015; 27:264-267.
32. Koh WP, Taylor MB, Hughes K, Chew SK, Fong CW, Phoon MC et al. Seroprevalence of IgG antibodies against Chlamydia pneumoniae in Chinese, Malays and Asian Indians in Singapore. Int J Epidemiol. 2002;31:1001-1007. doi: 10.1093/ije/31.5.1001
33. Mills GD, Lindeman JA, Fawcett JP, Herbison GP, Sears MR. Chlamydia pneumoniae serological status is not associated with asthma in children or young adults. Int J Epidemiol. 2000;29:280-284. doi: 10.1093/ije/29.2.280
34. Ferrari M, Poli A, Olivieri M, Verlato G, Tardivo S, Nicolis M et al. Respiratory symptoms, asthma, atopy and Chlamydia pneumoniae IgG antibodies in a general population sample of young adults. Infection. 2002;30:203-207. doi: 10.1007/s15010-002-2143-9
35. Nagy A, Keszei M, Kis Z, Kis Z, Budai I, Tölgyesi G et al. Chlamydophila pneumoniae infection status is dependent on the subtypes of asthma and allergy. Allergy Asthma Proc. 2007;28:58-63. doi: 10.2500/aap.2007.28.2957
36. Paldanius M, Juvonen R, Leinonen M, Bloigu A, Silvennoinen-Kassinen S, Saikku P. Asthmatic persons are prone to the persistence of Chlamydia pneumoniae antibodies. Diagn Microbiol Infect Dis. 2007;59:117-122. doi: 10.1016/j.diagmicrobio.2007.04.004
37. Chen Z, Salam MT, Alderete TL, Habre R, Bastain TM, Berhane K et al. Effects of Childhood Asthma on the Development of Obesity among School-aged Children. Am J Respir Crit Care Med. 2017;195:1181-1188. doi: 10.1164/rccm.201608-1691OC
38. Papoutsakis C, Chondronikola M, Antonogeorgos G, Papadakou E, Matziou V, Drakouli M et al. Associations between central obesity and asthma in children and adolescents: a case-control study. J Asthma. 2015;52:128-134. doi: 10.3109/02770903.2014.954291
39. Lang JE, Bunnell HT, Hossain MJ, Wysocki, T, Lima JJ, Finkel TH et al. Being overweight or obese and the development of asthma. Pediatrics. 2018;142:e20182119.doi: 10.1542/peds.2018-2119
40. Jiang D, Wang L, Bai C, Chen O. Association between abdominal obesity and asthma: a meta‑analysis. Allergy Asthma Clin Immunol. 2019; 15:16.doi: 10.1186/s13223-019-0333-6
41. Shan L-S, Zhou Q-L, Shang Y-X. Bidirectional Association Between Asthma and Obesity During Childhood and Adolescence: A Systematic Review and Meta-Analysis. Front. Pediatr. 2020; 8:576858.doi: 10.3389/fped.2020.576858
42. Porter M, Wegienka G, Havstad S, Nageotte CG, Johnson CC, Ownby DR et al. Relationship between childhood body mass index and young adult asthma. Ann Allergy Asthma Immunol. 2012; 109:408-411.e1. doi:10.1016/j.anai.2012.09.009
43. Visness CM, London SJ, Daniels JL, Kaufman JS, Yeatts KB, Siega-Riz AM et al. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999-2006. J Asthma. 2010;47:822-829. doi: 10.3109/02770903.2010.489388
44. Dowell SF, Peeling RW, Boman J, Carlone GM, Fields BS, Guarner J et al. Standardizing Chlamydia pneumoniae assays: recommendations from the Centers for Disease Control and Prevention (USA) and the Laboratory Centre for Disease Control (Canada). Clin Infect Dis. 2001; 33:492-503. doi: 10.1086/322632
45. Smith-Norowitz TA, Chotikanatis K, Erstein DP, Perlman J, Norowitz YM, Joks R et al. Chlamydia pneumoniae enhances the Th2 profile of stimulated peripheral blood mononuclear cells from asthmatic patients. Hum Immunol. 2016; 77:382-388. doi: 10.1016/j.humimm.2016.02.010
46. Smith-Norowitz TA, Huang Y, Loeffler J, Klein E, Norowitz YM, Hammerschlag MR et al. Azithromycin decreases Chlamydia pneumoniae-mediated Interleukin-4 responses but not Immunoglobulin E responses. PLoS ONE. 2020; 8:15:e0234413.doi: 10.1371/journal.pone.0234413