AN UPDATE ON TOXICITY OF THERAPEUTIC RADIONUCLIDES
Main Article Content
Abstract
Targeted radiotherapy is an evolving and promising modality of cancer treatment. Among the many advantages of this approach are its selectiveness in delivering the radiation to the target, relatively less severe and infrequent side effects, and the possibility of assessing the uptake by the tumor prior to the therapy. A number of radionuclides, such as iodine-131 (131I), phosphorus-32 (32P), strontium-90 (90Sr), and yttrium-90 (90Y), have been used successfully for the treatment of many benign and malignant disorders. The toxicity to radionuclides has come into vogue with its increasing utilization for multiple indications. Short term hematological toxicities include cytopenias and long term hematological toxicities include myeloid neoplasms. Non hematological toxicities commonly include renal and hepatotoxicity and long term toxicities like gonadal toxicity. This review focuses on the toxicities which need to be monitored during use of therapeutic radionuclides.

INTRODUCTION
Theranostics is a revolutionary approach that promises improved therapy selection on the basis of specific molecular features of disease, greater predictive power for adverse effects due to improved patient specific absorbed dose estimates, and new ways to objectively monitor therapy response. The history of radionuclide therapy can be traced back to the early 1900s, after the discovery of radioactivity by Henri Becquerel and Marie Curie. Radionuclide based targeted therapies have emerged as an effective mode of cancer treatment in the recent decades. Specific physical characteristics of Targeted Radionuclide Therapies (TRT) (heterogeneous and mixed irradiation, protracted exposure and low absorbed dose rate) differ from those of conventional EBRT (homogeneous irradiation, short exposure, and high absorbed dose rate), and, consequently the response of irradiated tissues might be different1.
An ideal radiopharmaceutical for therapeutic purposes should:
● Act exclusively on cancer cells
● Reach all the cancer cells wherever they are localized
● Leave healthy tissues and organs unhurt while bringing maximum doses of radiation to the tumour
● Eliminate malignant tumour cells with great effectiveness
Many therapeutic radionuclides are in use for multiple indications. Hence the question of toxicity, both short term and long term, has come into picture. In this article we try to focus on toxicities of radionuclides in terms of its classification, the risk of toxicity and how to mitigate them.
RADIONUCLIDES IN ONCOLOGY
There have been vast advances in the field of TRT in oncology. They are being used in various modes and forms for both therapeutic and diagnostic purposes. Table 1 gives a short list of various radionuclide agents used in the field of oncology.
Table 1 Commonly used radionuclide in oncology (therapeutic and diagnostic)2
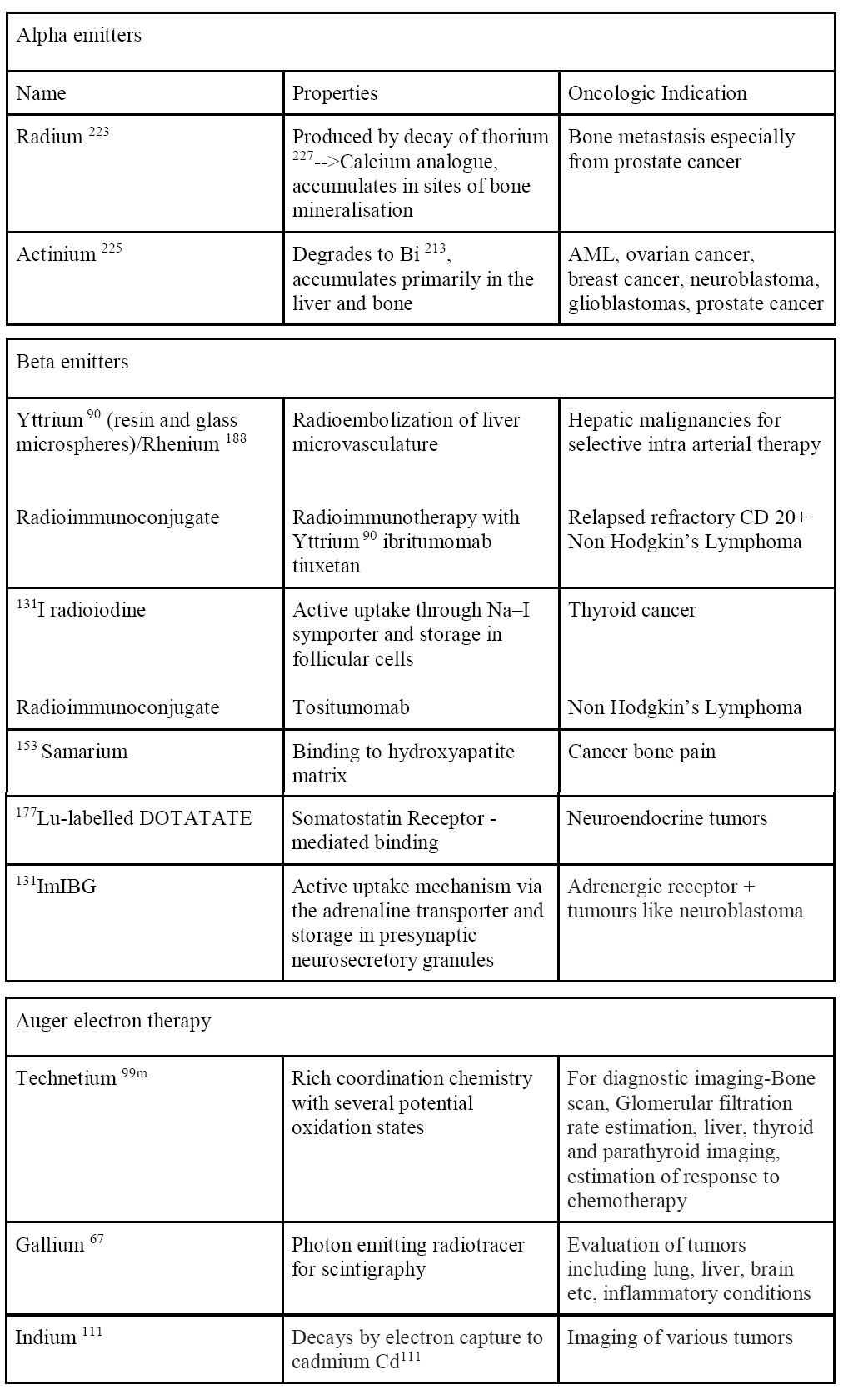
Therapeutic radionuclides are divided into energetic particles which include alpha emitters and beta emitters and non energetic particles. The selection of the appropriate radionuclide depends on its decay properties, specifically, emission characteristics and physical half-life. The radionuclides most commonly used in therapy emit particles with a low penetration range and high linear energy transfer (LET), leading to high ionization in the uptake site. A suitable range of the physical half-life for therapeutic radionuclides is between 6 hours and 7 days3. A very short physical half-life limits the delivery flexibility and is very impractical, while a long half-life allows for the retention of the radiation dose in the patient and exposes surrounding people for a longer period4.
The treatment of bulky tumors by radionuclides that emit high energy alpha or beta particles is the preferred approach; however, for the eradication of small clusters of cancer cells or small tumor deposits, radionuclides that emit Auger electrons are considered to be beneficial because of their high level of cytotoxicity and short-range biological effectiveness5.
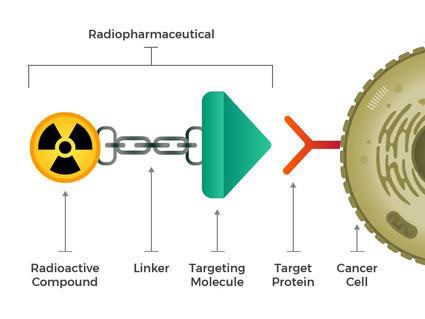
Structure of Targeted Radionuclide Therapy
The theranostic principle in nuclear medicine involves combining diagnostic imaging and therapy with the same molecule, which is radio-labeled differently, or administered in other dosages. The image shows a simplified model of a radiopharmaceutical, which consists of a binding molecule that binds the target, and a linking molecule, which binds the radioisotope. examples of such theranostic molecules are DOTA-TOC, DOTA-TATe, and 617PSMA 6.
MECHANISM OF ACTION
The distribution of therapeutic radiopharmaceuticals within a targeted solid tumor is not homogeneous7. This is mainly a result of:
(i) The radio-labeled molecules to penetrate non-uniformly within a solid tumor mass
(ii) The high interstitial pressure of solid tumors; and/or
(iii) Differences in the binding-site densities of tumor cells.
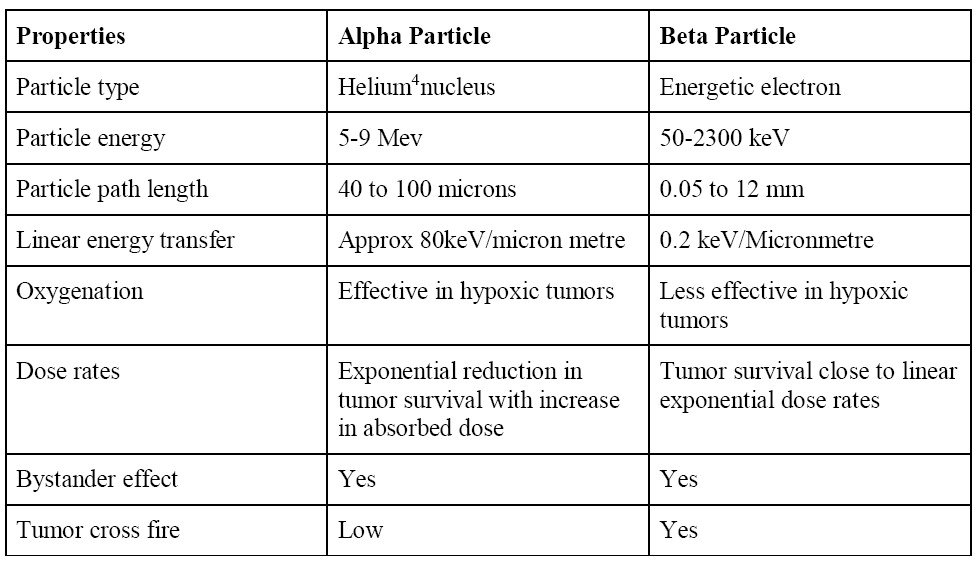
Energetic particles
These include alpha emitters and beta emitters.
The probability of the energetic particles traversing the targeted cell nucleus depends on:
(i) The position of the decaying atom vis-à-vis the nucleus – specifically nuclear DNA – of the targeted tumor cell
(ii) Distance from the tumor cell nucleus
(iii) Radius of the latter
Table 2 Differences between alpha and beta particles7
Non Energetic particles
During the decay of certain radioactive atoms, a vacancy is formed as a consequence of electron capture (EC). The transition results in the emission of characteristic Auger electrons. In an atom undergoing EC, on average, 5–30 Auger electrons with energies ranging from a few eV to approximately 1 keV are emitted. These are light, negatively charged particles that travel in contorted paths and their range in water is up to ~0.5 μm and result in multiple ionizations at the decay site. Thus, the short range of Auger electrons necessitates the close proximity to the target (tumour) for its radio-therapeutic effectiveness8.
MECHANISM OF TOXICITY TO RADIONUCLIDE THERAPY
A definition of toxicity hazard for a radionuclide is the probability that injury may be caused by the manner in which the radionuclide is used9. The toxicity to radionuclide is mostly related to the associated relative biological efficiency (RBE) dose, which is defined as the ratio of the doses required by two different radiations to cause the same level of effect10. The RBE of a given type of radiation will vary with particle type and energy, dose, dose per fraction, degree of oxygenation, cell or tissue type, biological endpoint, etc.
The types of radiation emitted, and their energies, have been well-established for most radionuclides and the disintegration rate of any radioactive sample can be measured. Therefore, if the concentration of a radionuclide by a body organ of known mass can be determined from experimental measurement, then the dose in rads delivered to the organ by the radionuclide can be calculated11. The product of RBE x dose (rad) is proportional to the risk of biological damage for all types of radiation9.
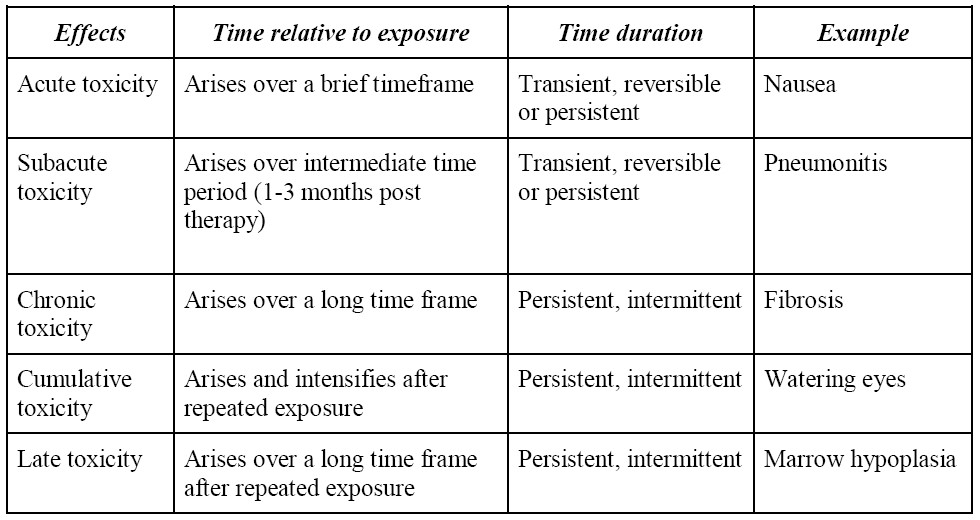
Time frame of toxicity
The toxicity to radionuclides can be classified into either acute or chronic. Response and toxicity prediction is essential for the rational implementation of cancer treatment using radionuclide therapy. As mentioned earlier, the biological effects of radionuclide therapy are mediated by a well-defined physical quantity, the absorbed dose, which is defined as the energy absorbed per unit mass of tissue2.
Standardised capture and reporting of toxicity
Existing methods like the NCI's Common Terminology Criteria for Adverse Events (CTCAE, version 5) are reliable and accurate for describing toxicities on a five-point scale based on clinical criteria. The NCI recognizes five discrete categories for any given CTCAE term that radiopharmaceutical-attributed toxicity must fit 12:
(A) laboratory/biomarker based toxicity that requires equipment to detect (like anemia, leukopenia, neutropenia, or thrombocytopenia)
(B) observable/measurable toxicity that requires technical training to delineate (like eye examination for tearing caused by corneal or limbic irritation)
(C) primarily subjective toxicity without observable components (like radiation-induced nausea)
(D) primarily subjective toxicity with observable components (like radiation-induced diarrhea)
(E) primarily observable toxicity with subjective components (like radiation-induced alopecia)
Table 3: Time frame to toxicity13,14
Risk factors for toxicity
The risk factors for radionuclide toxicity may be multifactorial but are unclear.
A patient who has received any form of cancer therapy (chemotherapy or radiotherapy) is deemed to have more toxicity due to poor bone marrow reserve; hence, toxicity may be attributed to pre-exposure to these agents.
For the kidney, these factors might include long-standing and poorly controlled hypertension or diabetes15.
Similarly, advanced age is also a risk factor for toxicity due altered organ functions. The role of clinical factors for predicting toxicity in NETs (neuroendocrine tumours) is unclear.
Identifying intrinsic susceptibility to radiation exposure is a key need that is unmet in the field of radiation oncobiology, to reduce severe, unpredictable effects in the long-term.
Seven Single Nucleotide Polymorphisms (SNPs) have been associated with late effects of radiotherapy. Genes range from TGFβ1 (growth factor signaling) to SLC36A4 (high affinity amino acid transporter) and are associated with endpoints related to toxicity, including fibrosis and overall toxicity16.
Gene expression profiling has also been used to study these toxic effects. Although these genes have been defined for external radiotherapy, they represent potential candidate factors for toxicity related to PRRT (peptide receptor radionuclide therapy17).
Proper monitoring of liver function and renal function tests are paramount during delivery of radionuclides.
Toxicities of commonly used radionuclides
Most of the toxicities with radionuclide are related to those associated with radiotherapy. Most studied among them are related to Peptide Receptor Radionuclide Therapy (PRRT). Hematologic and renal toxicities are dose limiting for radionuclide therapy.
Acute and subacute toxicity
Most common acute toxicities include
Nausea and Vomiting - Anticipatory, acute and delayed vomiting have been described with radionuclide therapy as with radiation. The pathophysiology of radiation-induced emesis is complex. It has been suggested that both serotonin levels and the abdomen play important roles in radiation-induced nausea and vomiting18.
Fatigue - Fatigue is associated with all forms of cancer therapy. The mechanism is complex. The recent evidence suggests it is related to mitochondrial dysfunction. The individual’s inflammatory response is another mechanism that is proposed to contribute to fatigue.19
Xerostomia - Radiation induced xerostomia and hyposalivation are multifactorial. The primary cause of irreversible hyposalivation is loss or impairment of acinar cells and their progressive replacement by connective tissue and fibrosis20. The frequency and extent of the resulting symptoms are dependent on the absorbed dose and the isotope used.
Hematological toxicity
Acute hematological toxicities include cytopenias. Acute toxicity manifested as modest self-limited grade 3/4 toxicity (CTCAE or WHO), most often affecting platelets, white blood cells (WBC), and, finally hemoglobin. Lymphopenia is also characteristic but the exact mechanism is not known. This is commonly observed during the first cycle of treatment, with the lowest nadir predictive of time taken for recovery during the first cycle of treatment21. Toxicity manifesting early is easily managed with dose modification or therapy cessation and was ameliorated by appropriate patient selection.
Renal toxicity
The kidneys have been considered as the “critical organ” because of the predominant glomerular filtration, tubular reabsorption and retention of the tracer by the proximal tubules. Severe nephrotoxicity depends on several factors, such as the amount of the single and cumulative administered radiopharmaceutical activity, treatment interval between the cycles, radioisotope which was preferred, renal absorbed dose, presence of patients’ risk factors, and features of renal protection22. Studies on the mechanism and localization demonstrate that renal uptake of radio-labeled somatostatin analogues largely depends on the megalin/cubulin system in the proximal tubule cells. Thus, methods are needed that interfere with this reabsorption pathway to achieve kidney protection23.
Hepatotoxicity
Hepatotoxicity has not been completely studied with radionuclide therapy. There have been case series and reports of hepatotoxicity occurring in metastatic neuroendocrine tumors post PRRT.
Radiation-induced hepatitis is a subacute toxicity and is thought to likely to occur when previous dose radiation has been delivered as a cumulative effect24.
There have also been case series studies of hepatotoxicity following transarterial radio-embolization with Yttrium 90 microspheres and usually occurs as subacute to late toxicity, but assessment is challenging as it is usually used in a palliative setting25,26.
Chronic toxicity
Hematological toxicity
Cumulative toxicity from radionuclides can result in bone marrow failure syndromes and malignancies27. Risk factors such as use of alkylating agents, metastatic disease to the bone, prior radiation, and others are associated with development of therapy related myeloid neoplasms28,29 (t-MN). There have also been studies suggesting that early occurrence of cytopenias is related to development of hematological malignancies. However, these factors have not been consistently implicated across studies. Recent reports suggest an association between preexisting somatic mutations and subsequent development of t-MN30.
Renal toxicity
Acute nephropathy can spontaneously recover or progress to chronic radiation nephropathy, which is characterized by volume loss and functional decline31. The latent period before the onset of the radiation-induced functional impairment depends on the cell turnover rate of the tissue. Kidney parenchyma is a slow turnover tissue, thus there is latency period before the functional damage is clinically detectable. Therefore, it is important to follow-up the renal function over a long period of time23.
Gonadal dysfunction
The major sources of irradiation to the gonads are from circulating radioactive particles and its accumulation in the bladder and rectum. Reduction in sperm count and damage to germinal epithelium are known to occur with radionuclide therapy which can lead to infertility in rare cases32.
Side effects of specific commonly used radionuclides
Peptide receptor radionuclide therapy
In a review of 2225 patients treated with PRRT, short-term myelotoxicity was observed in 221 patients (10%), occurring in 213 of 2104 patients treated with PRRT monotherapy and 8 of 121 patients treated with PRRT combined with chemotherapy21.
In another study by Bergesma et al, The prevalence of therapy-related persistent hematological dysfunction after PRRT with 177Lu-DOTATATE in GEP-NET patients was 3.7% implying a RR of 2.7. The median latency time to disease development was 41 months33.
In the phase 3 netter trial with lutetium 177 therapy in gut neuroendocrine tumors, the most common adverse events were nausea and vomiting and then fatigue and asthenia. Grade 3 or 4 neutropenia, thrombocytopenia, and lymphopenia were reported in 1%, 2%, and 9% of patients34.
In a recent systematic review by Sonbol et al which included 28 articles and 7334 patients, it was reported that cumulative incidence of t-MN after PRRT is 2.61% (4.38%). This incidence appears to be higher than is reported in other malignant neoplasms like breast and gastrointestinal cancers. The median time for development of therapy related myeloid neoplasms 33.8 months to. Thus, the latency period more closely resembles the 1 to 3 year period seen after treatment with topoisomerase-2 inhibitors27.
There have been several studies which have looked at salivary toxicity due to radionuclides, especially with lutetium 177 therapy and PSMA therapy. Xerostomia higher than grade 1 occurred more frequently in patients receiving a higher number of fractions. Most patients reported recovery from xerostomia after a few weeks. The duration of the symptoms was longer after the second or third therapy in most cases35,36.
In one large institutional series of 1109 NET patients treated with 90YDOTATOC, 103 patients (9%) experienced severe permanent renal toxicity, and the initial kidney uptake was found to be predictive for kidney damage in multivariable analysis37.
A retrospective analysis of 807 patients treated with PRRT showed severe (grade 3/4) permanent nephrotoxicity was observed only in 1.5% of patients, and pre-existing nephrotoxic risk factors were shown to have a limited role in predicting PRRT-induced renal insufficiency28. Overall, end-stage renal failure following PRRT is extremely rare.
Radioiodine ablation therapy
Nausea, lacrimal gland dysfunction and altered taste have been known to occur with radioiodine therapy with increasing doses.
Radiation-induced depressed bone marrow function is manifested as a transient reduction in platelet and leukocyte counts may occur 3 to 5 weeks after 131I administration. It is a dose-dependent response that usually recovers within 6 months38. Persistent marrow depression, however, may be seen in patients with renal insufficiency39. Patients with multiple bone metastatic lesions treated with large cumulative administrations of 131I experience more severe marrow toxicity, which may lead to myelodysplasia or aplastic anemia40. Leukemia has been reported, primarily in patients with multiple bone metastatic tumors who receive >18.5 GBq (500 mCi) of radioiodine32,41.
The long-term toxicities of RAI include secondary primary malignancy 42,43 (SPM), sialadenitis, nasolacrimal duct obstruction and infertility39.
Permanent salivary gland damage after 131I manifesting as xerostomia or salivary gland swelling has also been reported. It may be associated with transient symptoms at the time of therapy, or which appear months or even years later44. Symptoms of dry mouth or swelling persisted for up to 2.5 years in 10% of one series. In another study, 43% had persistent salivary gland complaints 12 months after 131I administration45. A small increase in the incidence of salivary gland neoplasms years after 131I therapy has been reported. 46,47 Gonadal dysfunction also has been reported in both males and females after radioiodine therapy. Transient amenorrhea and menstrual irregularities occurred in 25% of women receiving 131I therapy for thyroid cancer in a previously published series.48 Onset was a few months after administration of 131I, and symptoms lasted from 4 to 10 months. However, permanent infertility has not been reported. Similarly in males, temporary damage to the germinal epithelium is reflected by a transient elevation of levels of follicle-stimulating hormone, with normalization by 9 to 12 months after the last 131I administration.32
The cumulative dose of 131I correlated with the risk of bone, soft tissue, colorectal, and salivary gland cancers. In a meta-analysis that included 2 multicenter studies, the relative risk of leukemia in thyroid cancer survivors treated with 131I was 2.5 49 (95% confidence interval, 1.13 to 5.53; P = .024)
In a more recent meta-analysis, the risk ratio of any Secondary Neoplasms in RAI-treated TC patients was 0.98 ([confidence interval (CI) 0.76-1.27]. The pooled risk ratio for any neoplasm, adjusted for confounders, was 1.16 In secondary analyses examining specific neoplasm,, although relatively rare, the risk of subsequent leukemia was increased.50
Radium 223 therapy
In the phase 3 alsympca trial of metastatic prostate cancer treated with radium 223, common toxicities noted were nausea, bone pain, and anemia. Grade 3 or 4 hematological side effects observed were anemia (13%) , neutropenia (2%), and thrombocytopenia(6%).51
McKay et al evaluated 135 patients with mCRPC, 7 patients discontinued Ra-223 early for toxicity, and the independent predictors of therapy completion in a multivariable analysis included previous treatment with sipuleucel-T, hemoglobin, and ANC greater than the lower limit of normal.52
In a post hoc analysis of alsympca trial, baseline characteristics of patients in the Ra-223 safety arm significantly associated with the development of grade 2 to 4 anemia were the extent of disease, higher PSA levels, higher total ALP levels, and lower baseline hemoglobin levels.53
Recently, the US FDA conducted an adverse event reporting analysis and found 2182 radium223 cases associated with AE(s) from 2013 to 2018, as part of post marketing surveillance.The results of disproportionality analysis, conducted in a heterogenous group of patients, revealed strong signals for multiple hematologic AEs (anemia, thrombocytopenia, pancytopenia/bone marrow failure, and leukopenia). The gastrointestinal toxicities which include diarrhea and nausea were also commonly reported.54
There have been very rare case reports of secondary malignancies post radium 223 therapy,but longer follow up is required for a more specific recommendation in this regard.
MIBG therapy In a retrospective analysis of 66 neuroblastoma patients treated with MIBG therapy,the main grade 4 toxicity observed was haematological, occurring in stage 4 patients, after the first and second 131I-MIBG therapies. Nausea, vomiting and fever were common toxicities which occurred post infusion55.
Infections have also been reported during and after 131I-MIBG therapy in heavily pretreated patients and in patients treated with myeloablative 131I-MIBG therapy. Matthay et al. found infectious events (grade 3 or 4) in 10.9 % of patients with refractory neuroblastoma56.
In another study of 22 patients of neuroendocrine tumors (NET) treated with MIBG, toxicity was confined to transient myelosuppression of grade 3 or 4 in 15.3% (leukopenia) and 7.6% (thrombocytopenia) and a late event of myelodysplastic syndrome, after a cumulative administered activity of 66.6 GBq. The most frequent non hematologic side effect was mild nausea (grade 1 or 2), which was observed in 28% of administered cycles57.
In another recent study 68 patients of advanced paraganglioma and pheochromocytoma treated with high specific activity MIBG, most common treatment-emergent adverse events were nausea, myelosuppression, and fatigue. Sixty‐one patients (90%) experienced hematologic AEs, which were grade 3 or 4 AEs or SAEs in 49 (72%) of these patients58.
Drug induced hypertension and thyroid dysfunction has also been reported with MIBG therapy. Cases of late onset leukemia and myelodysplastic syndromes have also been reported post MIBG therapy59.
Yttrium 90 therapy
An increased incidence of cirrhosis or fibrosis following 90Y for NET has been reported in several analyses60. The rate doubled in those treated with whole-liver infusion. Radiation-induced hepatic fibrosis has also been noted61. There have been few reports of fatal toxicities in patients with metastatic NET following radioembolization. Whitney. report an episode of hepatic failure one month following radioembolization62. Su et al. report et al death from hepatic failure in 2 of patients in the absence of disease progression or subsequent therapies, while 6 additional patients died of liver failure in the setting of disease progression and subsequent exposure to potentially hepatotoxic systemic therapies63.
The most common AEs with 90Y-ibritumomab tiuxetan are hematologic toxicities as per various prospective and retrospective studies64. Dosimetric analysis demonstrated that radiation exposure with 90Y-ibritumomab tiuxetan consolidation was within safe limits both to normal organs and to red marrow. Incidence of second malignancies has also been reported around 2.5%. Fatigue and thyroid dysfunction have also been reported65.
Mitigating side effects
The mainstay of avoiding excess side effects is to calculate the exact dose using standardised dosimetry calculators.
For radioimmunotherapy, the normal organ dose limitations depend mainly on the dose absorbed by the bone marrow. One way to avoid unnecessary bone marrow exposure is to ensure a rapid clearance from blood, for instance by administering small molecules, such as bivalent or monovalent antibody fragments instead of intact IgG66.
Preventive strategies have not been successful in mitigating the side effects of salivary gland function (local cooling, lemon juice and vitamin C, or a displacement strategy using PMPA [2-(phosphonomethyl)pentane-1,5-dioic acid]) 20. Salivary gland toxicity is reported to be low when low-dose 131I ablation is used. In one study47, pain and tenderness over the salivary glands occurred in only 1.78% of patients receiving low-dose (1.48 GBq) 131I. Maximal reactions were experienced 24–48 h after therapy and all symptoms except xerostomia resolved within 1 week. Amifostine, which acts as a scavenger of oxygen-free radicals that mediate radiation-induced tissue damage, may help to reduce salivary gland damage 67.
Co-infusion of competitive inhibitors of re-absorption also interferes with the interaction of peptides with renal endocytic receptors; co-infusion of basic amino acids is currently used for kidney protection in clinical PRRT. Patient specific dosimetry may be helpful in minimizing the renal absorbed dose while maximizing the tumor dose. In addition, close and accurate renal function monitoring using more precise methods, rather than plasma creatinine levels, is essential to diagnose the early renal functional changes and to follow-up the renal function during the treatment22.
Avoidance of gonadal toxicity can be achieved by preventing accumulation of radioactivity in the bladder or rectum. Advice regarding excess fluid intake and emptying the bladder frequently should be explained. Constipation should be avoided and laxatives may be used32. Male patients likely to require repeated doses of radioiodine (cumulative dose >14 GBq) should be offered sperm banking because of the potential for low sperm counts68.
CONCLUSION
Radionuclide therapy has opened new avenues for treatment and diagnostics in malignancies. In general, the toxicity risk seems to depend on the characteristics of the molecule, such as the molecular weight, electric charges and clearance pathways and the chemical and physical characteristics. Many new radionuclides for various indications are on the horizon; hence, their use is likely to escalate. They are generally considered safe. Many of the toxicities are similar to those associated with traditional radiation therapy. One should be aware of the long term toxic effects of these radionuclides which will come into picture on further long term follow ups.
Article Details
The Medical Research Archives grants authors the right to publish and reproduce the unrevised contribution in whole or in part at any time and in any form for any scholarly non-commercial purpose with the condition that all publications of the contribution include a full citation to the journal as published by the Medical Research Archives.
References
2) Sgouros G, Bodei L, McDevitt MR, Nedrow JR. Radiopharmaceutical therapy in cancer: clinical advances and challenges [published correction appears in Nat Rev Drug Discov. 2020 Sep 7;:]. Nat Rev Drug Discov. 2020;19(9):589-608. doi:10.1038/s41573-020-0073-9
3) Qaim SM, Tarkanyi F, Capote R. Nuclear data for the production of therapeutic radionuclides. Vienna, Austria: International Atomic Energy Agency; 2011
4) Yeong CH, Cheng MH, Ng KH. Therapeutic radionuclides in nuclear medicine: current and future prospects. J Zhejiang Univ Sci B. 2014;15(10):845-863. doi:10.1631/jzus.B1400131
5) Ersahin D, Doddamane I, Cheng D. Targeted radionuclide therapy. Cancers (Basel). 2011;3(4):3838-3855. Published 2011 Oct 11. doi:10.3390/cancers3043838
6) Yordanova A, Eppard E, Kürpig S, et al. Theranostics in nuclear medicine practice. Onco Targets Ther. 2017;10:4821-4828. Published 2017 Oct 3. doi:10.2147/OTT.S140671
7) Kassis AI. Therapeutic radionuclides: biophysical and radiobiologic principles. Semin Nucl Med. 2008;38(5):358-366. doi:10.1053/j.semnuclmed.2008.05.002
8) Kassis AI. The amazing world of auger electrons. Int J Radiat Biol. 2004;80(11-12):789-803. doi:10.1080/09553000400017663
9) International Atomic Energy Agency, A Basic Toxicity Classification of Radionuclides, Technical Reports Series No. 15, IAEA, Vienna
10) Hobbs RF, Howell RW, Song H, Baechler S, Sgouros G. Redefining relative biological effectiveness in the context of the EQDX formalism: implications for alpha-particle emitter therapy. Radiat Res. 2014;181(1):90-98. doi:10.1667/RR13483.1
11) Fowler JF. 21 years of biologically effective dose. Br J Radiol. 2010;83(991):554-568. doi:10.1259/bjr/31372149
12) Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014;106(9):dju244. Published 2014 Sep 29. doi:10.1093/jnci/dju244
13) Kunos CA, Capala J, Finnigan S, Smith GL, Ivy SP. Radiopharmaceuticals for Relapsed or Refractory Ovarian Cancers. Front Oncol. 2019;9:180. Published 2019 Mar 26. doi:10.3389/fonc.2019.00180
14) Thanarajasingam G, Minasian LM, Baron F, et al. Beyond maximum grade: modernising the assessment and reporting of adverse events in haematological malignancies [published correction appears in Lancet Haematol. 2019 Mar;6(3):e121]. Lancet Haematol. 2018;5(11):e563-e598. doi:10.1016/S2352-3026(18)30051-6
15) Lambert B, Cybulla M, Weiner SM, et al. Renal toxicity after radionuclide therapy. Radiat Res. 2004;161(5):607-611. doi:10.1667/rr3105
16) Kerns SL, Ostrer H, Rosenstein BS. Radiogenomics: using genetics to identify cancer patients at risk for development of adverse effects following radiotherapy. Cancer Discov. 2014;4(2):155-165. doi:10.1158/2159-8290.CD-13-0197
17) Bodei L, Schöder H, Baum RP, et al. Molecular profiling of neuroendocrine tumours to predict response and toxicity to peptide receptor radionuclide therapy. Lancet Oncol. 2020;21(9):e431-e443. doi:10.1016/S1470-2045(20)30323-5
18) Abdelsayed GG. Management of radiation-induced nausea and vomiting. Exp Hematol. 2007;35(4 Suppl 1):34-36. doi:10.1016/j.exphem.2007.01.010
19) Hsiao CP, Daly B, Saligan LN. The Etiology and management of radiotherapy-induced fatigue. Expert Rev Qual Life Cancer Care. 2016;1(4):323-328. doi:10.1080/23809000.2016.1191948
20) Taïeb D, Foletti JM, Bardiès M, Rocchi P, Hicks RJ, Haberkorn U. PSMA-Targeted Radionuclide Therapy and Salivary Gland Toxicity: Why Does It Matter?. J Nucl Med. 2018;59(5):747-748. doi:10.2967/jnumed.118.207993
21) Kesavan M, Turner JH. Myelotoxicity of Peptide Receptor Radionuclide Therapy of Neuroendocrine Tumors: A Decade of Experience. Cancer Biother Radiopharm. 2016;31(6):189-198. doi:10.1089/cbr.2016.2035
22) Vegt E, de Jong M, Wetzels JF, et al. Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention. J Nucl Med. 2010;51(7):1049-1058. doi:10.2967/jnumed.110.075101
23) Erbas B, Tuncel M. Renal Function Assessment During Peptide Receptor Radionuclide Therapy. Semin Nucl Med. 2016;46(5):462-478. doi:10.1053/j.semnuclmed.2016.04.006
24) Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S94-S100. doi:10.1016/j.ijrobp.2009.06.092
25) Currie BM, Hoteit MA, Ben-Josef E, Nadolski GJ, Soulen MC. Radioembolization-Induced Chronic Hepatotoxicity: A Single-Center Cohort Analysis. J Vasc Interv Radiol. 2019;30(12):1915-1923. doi:10.1016/j.jvir.2019.06.003
26) Su YK, Mackey RV, Riaz A, et al. Long-Term Hepatotoxicity of Yttrium-90 Radioembolization as Treatment of Metastatic Neuroendocrine Tumor to the Liver. J Vasc Interv Radiol. 2017;28(11):1520-1526. doi:10.1016/j.jvir.2017.05.011
27) Sonbol MB, Halfdanarson TR, Hilal T. Assessment of Therapy-Related Myeloid Neoplasms in Patients With Neuroendocrine Tumors After Peptide Receptor Radionuclide Therapy: A Systematic Review. JAMA Oncol. 2020;6(7):1086-1092. doi:10.1001/jamaoncol.2020.0078
28) Bodei L, Kidd M, Paganelli G, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42(1):5-19. doi:10.1007/s00259-014-2893-5
29) Bergsma H, van Lom K, Raaijmakers MHGP, et al. Persistent Hematologic Dysfunction after Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE: Incidence, Course, and Predicting Factors in Patients with Gastroenteropancreatic Neuroendocrine Tumors. J Nucl Med. 2018;59(3):452-458. doi:10.2967/jnumed.117.189712
30) Takahashi K, Wang F, Kantarjian H, et al. Preleukaemic clonal haemopoiesis and risk of therapy-related myeloid neoplasms: a case-control study. Lancet Oncol. 2017;18(1):100-111. doi:10.1016/S1470-2045(16)30626-X
31) Van Binnebeek S, Baete K, Terwinghe C, et al. Significant impact of transient deterioration of renal function on dosimetry in PRRT. Ann Nucl Med. 2013;27(1):74-77. doi:10.1007/s12149-012-0651-y
32) Hyer SL, Newbold K, Harmer CL. Early and late toxicity of radioiodine therapy: detection and management. Endocr Pract. 2010;16(6):1064-1070. doi:10.4158/EP10170.RA
33) Bergsma H, Konijnenberg MW, Kam BL, et al. Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: prognostic factors, incidence and course. Eur J Nucl Med Mol Imaging. 2016;43(3):453-463. doi:10.1007/s00259-015-3193-4
34) Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376(2):125-135. doi:10.1056/NEJMoa1607427
35) Zechmann CM, Afshar-Oromieh A, Armor T, et al. Radiation dosimetry and first therapy results with a (124)I/ (131)I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41(7):1280-1292. doi:10.1007/s00259-014-2713-y
36) Afshar-Oromieh A, Haberkorn U, Zechmann C, et al. Repeated PSMA-targeting radioligand therapy of metastatic prostate cancer with 131I-MIP-1095. Eur J Nucl Med Mol Imaging. 2017;44(6):950-959. doi:10.1007/s00259-017-3665-9
37) Imhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29(17):2416-2423. doi:10.1200/JCO.2010.33.7873
38) Dorn R, Kopp J, Vogt H, Heidenreich P, Carroll RG, Gulec SA. Dosimetry-guided radioactive iodine treatment in patients with metastatic differentiated thyroid cancer: largest safe dose using a risk-adapted approach. J Nucl Med. 2003;44(3):451-456.
39) Andresen NS, Buatti JM, Tewfik HH, Pagedar NA, Anderson CM, Watkins JM. Radioiodine Ablation following Thyroidectomy for Differentiated Thyroid Cancer: Literature Review of Utility, Dose, and Toxicity. Eur Thyroid J. 2017;6(4):187-196. doi:10.1159/000468927
40) Alevizaki C, Molfetas M, Samartzis A, et al. Iodine 131 treatment for differentiated thyroid carcinoma in patients with end stage renal failure: dosimetric, radiation safety, and practical considerations. Hormones (Athens). 2006;5(4):276-287. doi:10.14310/horm.2002.11193
41) BENUA RS, CICALE NR, SONENBERG M, RAWSON RW. The relation of radioiodine dosimetry to results and complications in the treatment of metastatic thyroid cancer. Am J Roentgenol Radium Ther Nucl Med. 1962;87:171-182.
42) Brown AP, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, Tward JD. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93(2):504-515. doi:10.1210/jc.2007-1154
43) Teng CJ, Hu YW, Chen SC, et al. Use of Radioactive Iodine for Thyroid Cancer and Risk of Second Primary Malignancy: A Nationwide Population-Based Study. J Natl Cancer Inst. 2015;108(2):djv314. Published 2015 Nov 3. doi:10.1093/jnci/djv314
44) Allweiss P, Braunstein GD, Katz A, Waxman A. Sialadenitis following I-131 therapy for thyroid carcinoma: concise communication. J Nucl Med. 1984;25(7):755-758.
45) Alexander C, Bader JB, Schaefer A, Finke C, Kirsch CM. Intermediate and long-term side effects of high-dose radioiodine therapy for thyroid carcinoma. J Nucl Med. 1998;39(9):1551-1554.
46) Mandel SJ, Mandel L. Radioactive iodine and the salivary glands. Thyroid. 2003;13(3):265-271. doi:10.1089/105072503321582060
47) Hyer S, Kong A, Pratt B, Harmer C. Salivary gland toxicity after radioiodine therapy for thyroid cancer. Clin Oncol (R Coll Radiol). 2007;19(1):83-86. doi:10.1016/j.clon.2006.11.005
48) Vini L, Hyer S, Al-Saadi A, Pratt B, Harmer C. Prognosis for fertility and ovarian function after treatment with radioiodine for thyroid cancer. Postgrad Med J. 2002;78(916):92-93. doi:10.1136/pmj.78.916.92
49) Sawka AM, Thabane L, Parlea L, et al. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and meta-analysis. Thyroid. 2009;19(5):451-457. doi:10.1089/thy.2008.0392
50) Yu CY, Saeed O, Goldberg AS, et al. A Systematic Review and Meta-Analysis of Subsequent Malignant Neoplasm Risk After Radioactive Iodine Treatment of Thyroid Cancer. Thyroid. 2018;28(12):1662-1673. doi:10.1089/thy.2018.0244
51) Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223. doi:10.1056/NEJMoa1213755
52) McKay RR, Jacobus S, Fiorillo M, et al. Radium-223 Use in Clinical Practice and Variables Associated With Completion of Therapy. Clin Genitourin Cancer. 2017;15(2):e289-e298. doi:10.1016/j.clgc.2016.08.015
53) Vogelzang NJ, Coleman RE, Michalski JM, et al. Hematologic Safety of Radium-223 Dichloride: Baseline Prognostic Factors Associated With Myelosuppression in the ALSYMPCA Trial. Clin Genitourin Cancer. 2017;15(1):42-52.e8. doi:10.1016/j.clgc.2016.07.027
54) Huynh-Le MP, Shults RC, Connor MJ, Hattangadi-Gluth JA. Adverse Events Associated With Radium-223 in Metastatic Prostate Cancer: Disproportionality Analysis of FDA Data Reflecting Worldwide Utilization. Clin Genitourin Cancer. 2020;18(3):192-200.e2. doi:10.1016/j.clgc.2019.11.017
55) Bleeker G, Schoot RA, Caron HN, et al. Toxicity of upfront ¹³¹I-metaiodobenzylguanidine (¹³¹I-MIBG) therapy in newly diagnosed neuroblastoma patients: a retrospective analysis. Eur J Nucl Med Mol Imaging. 2013;40(11):1711-1717. doi:10.1007/s00259-013-2510-z
56) Matthay KK, Quach A, Huberty J, et al. Iodine-131--metaiodobenzylguanidine double infusion with autologous stem-cell rescue for neuroblastoma: a new approaches to neuroblastoma therapy phase I study. J Clin Oncol. 2009;27(7):1020-1025. doi:10.1200/JCO.2007.15.7628
57) Ezziddin S, Sabet A, Logvinski T, et al. Long-term outcome and toxicity after dose-intensified treatment with 131I-MIBG for advanced metastatic carcinoid tumors. J Nucl Med. 2013;54(12):2032-2038. doi:10.2967/jnumed.112.119313
58) Pryma DA, Chin BB, Noto RB, et al. Efficacy and Safety of High-Specific-Activity 131I-MIBG Therapy in Patients with Advanced Pheochromocytoma or Paraganglioma. J Nucl Med. 2019;60(5):623-630. doi:10.2967/jnumed.118.217463
59) Polishchuk AL, Dubois SG, Haas-Kogan D, Hawkins R, Matthay KK. Response, survival, and toxicity after iodine-131-metaiodobenzylguanidine therapy for neuroblastoma in preadolescents, adolescents, and adults. Cancer. 2011;117(18):4286-4293. doi:10.1002/cncr.25987
60) Zuckerman DA, Kennard RF, Roy A, Parikh PJ, Weiner AA. Outcomes and toxicity following Yttrium-90 radioembolization for hepatic metastases from neuroendocrine tumors-a single-institution experience. J Gastrointest Oncol. 2019;10(1):118-127. doi:10.21037/jgo.2018.10.05
61) Maker AV, August C, Maker VK, Weisenberg E. Hepatectomy After Yttrium-90 (Y90) Radioembolization-Induced Liver Fibrosis. J Gastrointest Surg. 2016;20(4):869-870. doi:10.1007/s11605-016-3077-3
62) Saxena A, Kapoor J, Meteling B, Morris DL, Bester L. Yttrium-90 radioembolization for unresectable, chemoresistant breast cancer liver metastases: a large single-center experience of 40 patients. Ann Surg Oncol. 2014;21(4):1296-1303. doi:10.1245/s10434-013-3436-1
63) Su YK, Mackey RV, Riaz A, et al. Long-Term Hepatotoxicity of Yttrium-90 Radioembolization as Treatment of Metastatic Neuroendocrine Tumor to the Liver. J Vasc Interv Radiol. 2017;28(11):1520-1526. doi:10.1016/j.jvir.2017.05.011
64) Witzig TE, White CA, Gordon LI, et al. Safety of yttrium-90 ibritumomab tiuxetan radioimmunotherapy for relapsed low-grade, follicular, or transformed non-hodgkin's lymphoma. J Clin Oncol. 2003;21(7):1263-1270. doi:10.1200/JCO.2003.08.043
65) Larson SM, Carrasquillo JA, Cheung NK, Press OW. Radioimmunotherapy of human tumours [published correction appears in Nat Rev Cancer. 2015 Aug;15(8):509]. Nat Rev Cancer. 2015;15(6):347-360. doi:10.1038/nrc3925
66) Akizawa H, Arano Y. Altering pharmacokinetics of radiolabeled antibodies by the interposition of metabolizable linkages: metabolizable linkers and pharmacokinetics of monoclonal antibodies. Q J Nucl Med. 2002;46:206–223.
67) Bohuslavizki KH, Brenner W, Klutmann S, et al. Radioprotection of salivary glands by amifostine in high-dose radioiodine therapy. J Nucl Med. 1998;39(7):1237-1242.
68) Krassas GE, Perros P. Thyroid disease and male reproductive function. J Endocrinol Invest. 2003;26(4):372-380. doi:0.1007/BF03345187